Pralsetinib
 | |
| Names | |
|---|---|
| Trade names | Gavreto |
| Other names | BLU-667 |
| |
| Clinical data | |
| Drug class | Tyrosine kinase inhibitor[1] |
| Main uses | Non-small cell lung cancer (NSCLC), thyroid cancer[1] |
| Side effects | Constipation, high blood pressure, tiredness, muscle pain, low blood cells, liver problems, low calcium[1] |
| Routes of use | By mouth |
| Typical dose | 400 mg OD[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620057 |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
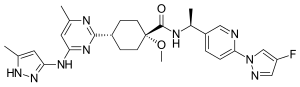
| Formula | C27H32FN9O2 |
| Molar mass | 533.612 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pralsetinib, sold under the brand name Gavreto, is a medication used to treat non-small cell lung cancer (NSCLC) and thyroid cancer.[1] It is used in advanced cases which are RET fusion-positive.[1] It is taken by mouth.[1]
Common side effects include constipation, high blood pressure, tiredness, muscle pain, low blood cells, liver problems, and low calcium.[1] Other side effects may include pneumonitis, bleeding, tumor lysis syndrome, and poor wound healing.[1] Use in pregnancy may harm the baby.[1] It is a tyrosine kinase inhibitor.[1]
Pralsetinib was approved for medical use in the United States in 2020.[1] In the United States it costs about 20,100 USD per month.[2] It is not approved for use in the United Kingdom as of 2021.[3]
Medical uses
Pralsetinib is indicated for the treatment of adults with metastatic RET fusion-positive non-small cell lung cancer (NSCLC) as detected by an FDA approved test.[4][5]
Dosage
It is generally taken at a dose of 400 mg once daily.[1]
History

Efficacy was investigated in a multicenter, open-label, multi-cohort clinical trial (ARROW, NCT03037385) with 220 participants aged 26-87 whose tumors had RET alterations.[4][5] Identification of RET gene alterations was prospectively determined in local laboratories using either next generation sequencing, fluorescence in situ hybridization, or other tests.[4] The main efficacy outcome measures were overall response rate (ORR) and response duration determined by a blinded independent review committee using RECIST 1.1.[4] The trial was conducted at sites in the United States, Europe and Asia.[5]
Efficacy for RET fusion-positive NSCLC was evaluated in 87 participants previously treated with platinum chemotherapy.[4] The ORR was 57% (95% CI: 46%, 68%); 80% of responding participants had responses lasting 6 months or longer.[4] Efficacy was also evaluated in 27 participants who never received systemic treatment.[4] The ORR for these participants was 70% (95% CI: 50%, 86%); 58% of responding participants had responses lasting 6 months or longer.[4]
The US Food and Drug Administration (FDA) granted the application for pralsetinib priority review, orphan drug, and breakthrough therapy designations[4]and granted approval of Gavreto to Blueprint Medicines.[4]
Society and culture
Legal status
On 16 September 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a conditional marketing authorization for the medicinal product Gavreto, intended for the treatment of people with rearranged during transfection (RET)-fusion positive non-small cell lung cancer (NSCLC).[7] The applicant for this medicinal product is Roche Registration GmbH.[7]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 "Gavreto- pralsetinib capsule". DailyMed. 9 September 2020. Archived from the original on 28 November 2020. Retrieved 24 September 2020.
- ↑ "Gavreto Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 29 October 2021.
- ↑ "Pralsetinib". SPS - Specialist Pharmacy Service. 4 July 2020. Archived from the original on 29 October 2021. Retrieved 29 October 2021.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 "FDA approves pralsetinib for lung cancer with RET gene fusions". U.S. Food and Drug Administration (FDA). 4 September 2020. Archived from the original on 9 September 2020. Retrieved 8 September 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 5.0 5.1 5.2 "Drug Trial Snapshot: Gavreto". U.S. Food and Drug Administration. 4 September 2020. Archived from the original on 25 October 2020. Retrieved 16 September 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Sun, Shi-Yong; Su, Chunxia (30 August 2022). Challenges and Opportunities of TKIs in the Treatment of NSCLC Patients With Uncommon Mutations. Frontiers Media SA. p. 9. ISBN 978-2-88976-866-0. Retrieved 25 November 2023.
- ↑ 7.0 7.1 "Gavreto: Pending EC decision". European Medicines Agency. 17 September 2021. Archived from the original on 17 September 2021. Retrieved 17 September 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- "Pralsetinib". NCI Drug Dictionary. National Cancer Institute. Archived from the original on 14 July 2020. Retrieved 24 September 2021.
- Clinical trial number NCT03037385 for "Phase 1/2 Study of the Highly-selective RET Inhibitor, Pralsetinib (BLU-667), in Patients With Thyroid Cancer, Non-Small Cell Lung Cancer, and Other Advanced Solid Tumors (ARROW)" at ClinicalTrials.gov
- "Understanding Metastatic RET Fusion-Positive Non-Small Cell Lung Cancer (NSCLC)" (PDF). Archived (PDF) from the original on 13 December 2020. Retrieved 24 September 2021.
- "Understanding Metastatic RET-Driven Thyroid Cancers" (PDF). Archived (PDF) from the original on 19 September 2020. Retrieved 24 September 2021.
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Drugs not assigned an ATC code
- Breakthrough therapy
- Orphan drugs
- Receptor tyrosine kinase inhibitors
- RTT