Poxviridae
| Poxviridae | |
|---|---|

| |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Varidnaviria |
| Kingdom: | Bamfordvirae |
| Phylum: | Nucleocytoviricota |
| Class: | Pokkesviricetes |
| Order: | Chitovirales |
| Family: | Poxviridae |
| Subfamilies | |
Poxviridae is a family of double-stranded DNA viruses. Vertebrates and arthropods serve as natural hosts. There are currently 83 species in this family, divided among 22 genera, which are divided into two subfamilies. Diseases associated with this family include smallpox.[1][2]
Four genera of poxviruses may infect humans: Orthopoxvirus, Parapoxvirus, Yatapoxvirus, Molluscipoxvirus. Orthopoxvirus: smallpox virus , vaccinia virus, cowpox virus, monkeypox virus; Parapoxvirus: orf virus, pseudocowpox, bovine papular stomatitis virus; Yatapoxvirus: tanapox virus, yaba monkey tumor virus; Molluscipoxvirus: molluscum contagiosum virus .The most common are vaccinia and molluscum contagiosum, but monkeypox infections are rising . The similarly named disease chickenpox is not a true poxvirus and is caused by the herpesvirus varicella zoster.[3][4][5][6]
Microbiology
Structure
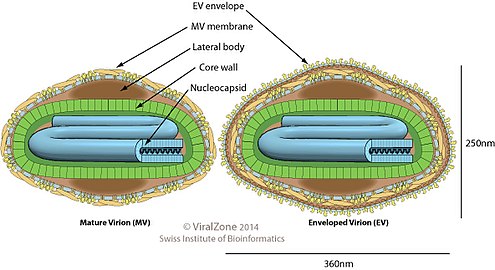
Poxviridae viral particles (virions) are generally enveloped (external enveloped virion), though the intracellular mature virion form of the virus, which contains different envelope, is also infectious. They vary in their shape depending upon the species but are generally shaped like a brick or as an oval form similar to a rounded brick because they are wrapped by the endoplasmic reticulum. The virion is exceptionally large, its size is around 200 nm in diameter and 300 nm in length and carries its genome in a single, linear, double-stranded segment of DNA.[7] By comparison, rhinoviruses are 1/10 as large as a typical Poxviridae virion.[8]
-
Poxviridae virion
-
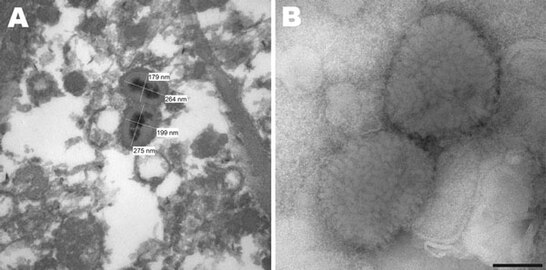
A) Electron micrograph of poxvirus particles in synovium of a big brown bat, northwestern United States. B) Negative staining of poxvirus particles in cell culture supernatant. Scale bar = 100 nm.
Genome
Phylogenetic analysis of 26 different chordopoxvirus genomes has shown that the central region of the genome is conserved and contains ~90 genes. [9] Of this group Avipoxvirus is the most divergent, the next most divergent is Molluscipoxvirus. Capripoxvirus, Leporipoxvirus, Suipoxvirus and Yatapoxvirus genera cluster together: Capripoxvirus and Suipoxvirus share a common ancestor and are distinct from the genus Orthopoxvirus. Within the Othopoxvirus genus Cowpox virus strain Brighton Red, Ectromelia virus and Monkeypox virus do not group closely with any other member. Variola virus and Camelpox virus form a subgroup. Vaccinia virus is most closely related to CPV-GRI-90.The GC-content of family member genomes differ considerably. Avipoxvirus, capripoxvirus, cervidpoxvirus, orthopoxvirus, suipoxvirus, yatapoxvirus and one Entomopox genus along with several other unclassified Entomopoxviruses have a low G+C content while others - Molluscipoxvirus, Orthopoxvirus, Parapoxvirus and some unclassified Chordopoxvirus - have a relatively high G+C content. The reasons for these differences are not known.[10][11][12][13]

Replication
Replication of the poxvirus involves several stages.[14] The virus first binds to a receptor on the host cell surface; the receptors for the poxvirus are thought to be glycosaminoglycans.After binding to the receptor, the virus enters the cell where it uncoats. Uncoating of the virus is a two step process.[1][15]
Firstly the outer membrane is removed as the particle enters the cell; secondly the virus particle fuses with the cellular membrane to release the core into the cytoplasm. The pox viral genes are expressed in two phases.The early genes encode the non-structural protein, including proteins necessary for replication of the viral genome, and are expressed before the genome is replicated. The late genes are expressed after the genome has been replicated and encode the structural proteins to make the virus particle. The assembly of the virus particle occurs in five stages of maturation that lead to the final exocytosis of the new enveloped virion. After the genome has been replicated, the immature virion assembles the A5 protein to create the intracellular mature virion. The protein aligns and the brick-shaped envelope of the intracellular enveloped virion. These particles are then fused to the cell plasma to form the cell-associated enveloped virion, which encounters the microtubules and prepares to exit the cell as an extracellular enveloped virion. The assembly of the virus particle occurs in the cytoplasm of the cell and is a complex process that is currently being researched to understand each stage in more depth. Considering the fact that this virus is large and complex, replication is relatively quick taking approximately 12 hours until the host cell dies by the release of viruses.[1][16][17][15]
The replication of poxvirus is unusual for a virus with double-stranded DNA genome because it occurs in the cytoplasm,although this is typical of other large DNA viruses. Poxvirus encodes its own machinery for genome transcription, a DNA dependent RNA polymerase,which makes replication in the cytoplasm possible. Most double-stranded DNA viruses require the host cell's DNA-dependent RNA polymerase to perform transcription. These host polymerases are found in the nucleus, and therefore most double-stranded DNA viruses carry out a part of their infection cycle within the host cell's nucleus.[18][19][20]
Vaccinia virus

The prototypical poxvirus is vaccinia virus, known for its role in the eradication of smallpox. The vaccinia virus is an effective tool for foreign protein expression, as it elicits a strong host immune-response. The vaccinia virus enters cells primarily by cell fusion, although currently the receptor responsible is unknown.[21][22]
Vaccinia contains three classes of genes: early, intermediate and late. These genes are transcribed by viral RNA polymerase and associated transcription factors. Vaccinia replicates its genome in the cytoplasm of infected cells, and after late-stage gene expression undergoes virion morphogenesis, which produces intracellular mature virions contained within an envelope membrane. The origin of the envelope membrane is still unknown. The intracellular mature virions are then transported to the Golgi apparatus where it is wrapped with an additional two membranes, becoming the intracellular enveloped virus. This is transported along cytoskeletal microtubules to reach the cell periphery, where it fuses with the plasma membrane to become the cell-associated enveloped virus. This triggers actin tails on cell surfaces or is released as external enveloped virion.[23][24][25][26]
Taxonomy
The species in the subfamily Chordopoxvirinae infect vertebrates and those in the subfamily Entomopoxvirinae infect insects. There are ten recognised genera in the Chordopoxvirinae and three in the Entomopoxvirinae.[27][28]
The following subfamilies and genera are recognized (-virinae denotes subfamily and -virus denotes genus):[2]
- Avipoxvirus
- Capripoxvirus
- Centapoxvirus
- Cervidpoxvirus
- Crocodylidpoxvirus
- Leporipoxvirus
- Macropopoxvirus
- Molluscipoxvirus
- Mustelpoxvirus
- Orthopoxvirus
- Oryzopoxvirus
- Parapoxvirus
- Pteropopoxvirus
- Salmonpoxvirus
- Sciuripoxvirus
- Suipoxvirus
- Vespertilionpoxvirus
- Yatapoxvirus
- Alphaentomopoxvirus
- Betaentomopoxvirus
- Deltaentomopoxvirus
- Diachasmimorpha entomopoxvirus
- Gammaentomopoxvirus
Both subfamilies also contain a number of unclassified species for which new genera may be created:
- Cotia virus of 2012 is an unusual chordopoxvirus that may belong to a new genus.[29] Cotia virus was assigned the new genus Oryzopoxvirus in 2019.[30] A Brazilian porcupinepox virus discovered in 2019 is closely related to the virus.[31]
- Two more chordopoxviruses are NY_014 and murmansk poxvirus. They are considered closely related to a "Yoka poxvirus".[32] ICTV classifies them under a genus Centapoxvirus, created 2016.[33]
Evolution

The ancestor of the poxviruses is not known but structural studies suggest it may have been an adenovirus or a species related to both the poxviruses and the adenoviruses.[34]
Based on the genome organisation and DNA replication mechanism a phylogenetic relationships may exist between the rudiviruses (Rudiviridae) and the large eukaryal DNA viruses: the African swine fever virus (Asfarviridae), Chlorella viruses (Phycodnaviridae) and poxviruses (Poxviridae).[35]
The mutation rate in poxvirus genomes has been estimated to be 0.9–1.2 x 10−6 substitutions per site per year.[36]
A second estimate puts this rate at 0.5–7 × 10−6 nucleotide substitutions per site per year.[37]
A third estimate places the rate at 4–6 × 10−6.[38]
The last common ancestor of the extant poxviruses that infect vertebrates existed 0.5 million years ago. The genus Avipoxvirus diverged from the ancestor 249 ± 69 thousand years ago. The ancestor of the genus Orthopoxvirus was next to diverge from the other clades at 0.3 million years ago. A second estimate of this divergence time places this event at 166,000 ± 43,000 years ago.[37] The division of the Orthopoxvirus into the extant genera occurred ~14,000 years ago. The genus Leporipoxvirus diverged ~137,000 ± 35,000 years ago. This was followed by the ancestor of the genus Yatapoxvirus. The last common ancestor of the Capripoxvirus and Suipoxvirus diverged 111,000 ± 29,000 years ago.[37][38]
An isolate from a fish – salmon gill poxvirus – appears to be the earliest branch in the Chordopoxvirinae.[39] A new systematic has been proposed recently after findings of a new squirrel poxvirus in Berlin, Germany.[40]
Smallpox
The date of the appearance of smallpox is not settled, though it most likely evolved from a rodent virus between 68,000 and 16,000 years ago, the wide range of dates is due to the different records used to calibrate the molecular clock. One clade was the variola major strains which spread from Asia between 400 and 1,600 years ago. A second clade included both alastrim minor described from the American continents and isolates from West Africa which diverged from an ancestral strain between 1,400 and 6,300 years before present. This clade further diverged into two subclades at least 800 years ago.[41][42]
A second estimate has placed the separation of variola from Taterapox at 3000–4000 years ago.[38] This is consistent with archaeological and historical evidence regarding the appearance of smallpox as a human disease which suggests a relatively recent origin. However, if the mutation rate is assumed to be similar to that of the herpesviruses the divergence date between variola from Taterapox has been estimated to be 50,000 years ago.[38]
Signs and symptoms
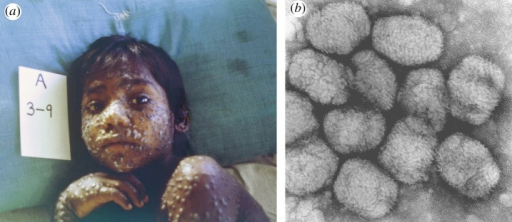
The presentation of smallpox is fever of at least 38.3 °C (101 °F), muscle pain, malaise, headache and fatigue, as well as nausea and vomiting and backache. Visible lesions appear on mucous membranes of the mouth, tongue, palate, and throat[43][44]
Diagnosis
The diagnosis of smallpox is defined as an illness with acute onset of fever equal to or greater than 38.3 °C (101 °F) followed by a rash which is firm, deep-seated vesicles or pustules in the same stage of development without other apparent cause. Smallpox is confirmed using laboratory tests.[45][46]
Treatment
Smallpox vaccination within three days of exposure will prevent or significantly lessen the severity of smallpox symptoms in the vast majority of people. Vaccination four to seven days after exposure can offer some protection from disease or may modify the severity of disease.[47] In July 2018, the Food and Drug Administration approved tecovirimat, the first drug approved for treatment of smallpox.[48]
Eradication
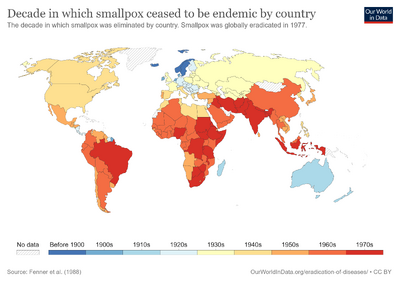
The last major European outbreak of smallpox was in 1972 in Yugoslavia, after a pilgrim from Kosovo returned from the Middle East, where he had contracted the virus. The epidemic infected 175 people, causing 35 deaths. Authorities declared martial law, enforced quarantine, and undertook widespread re-vaccination of the population, enlisting the help of the WHO. In two months, the outbreak was over.[49] By the end of 1975, smallpox persisted only in the Horn of Africa. Conditions were very difficult in Ethiopia and Somalia, where there were few roads. Civil war, famine, and refugees made the task even more difficult. An intensive surveillance and containment and vaccination program was undertaken in these countries in early and mid-1977, under the direction of Australian microbiologist Frank Fenner. As the campaign neared its goal, Fenner and his team played an important role in verifying eradication.[50] The last naturally occurring case of the more deadly variola major had been detected in October 1975 in a three-year-old Bangladeshi girl, Rahima Banu.[51]
The global eradication of smallpox was certified, based on intense verification activities, by a commission of eminent scientists on 9 December 1979 and subsequently endorsed by the World Health Assembly on 8 May 1980.[52][53]
History

Diseases caused by pox viruses, especially smallpox, have been known about for centuries. One of the earliest suspected cases is that of Egyptian pharaoh Ramses V who is thought to have died from smallpox circa 1150 years BCE.[56][57]
Smallpox was thought to have been transferred to Europe around the early 11th century and then to the Americas in the early 17th century, resulting in the deaths of 3.2 million Aztecs within two years of introduction. This death toll can be attributed to the indigenous population's complete lack of exposure to the virus over millennia.A century after Edward Jenner showed that the less potent cowpox could be used to effectively vaccinate against the more deadly smallpox, a worldwide effort to vaccinate everyone against smallpox began with the ultimate goal to rid the world of the plague-like epidemic. The last case of endemic smallpox occurred in Somalia in 1977. Extensive searches over two years detected no further cases, and in 1979 the World Health Organization (WHO) declared the disease officially eradicated.[58][54][55][59]
In 1986, all virus samples were destroyed or transferred to two approved WHO reference labs: at the headquarters of the federal Centers for Disease Control and Prevention (the C.D.C.) in Atlanta, Georgia (the United States) and at the Institute of Virus Preparations in Moscow.[60]
After September 11, 2001 the American and UK governments have had increased concern over the use of smallpox, or a smallpox-like disease, in bioterrorism. However, several poxviruses including vaccinia virus, myxoma virus, tanapox virus and raccoon pox virus are currently being investigated for their therapeutic potential in various human cancers in preclinical and clinical studies.[61][62][63]
Etymology
The name of the family, Poxviridae, is a legacy of the original grouping of viruses associated with diseases that produced poxes in the skin. Modern viral classification is based on phenotypic characteristics; morphology, nucleic acid type, mode of replication, host organisms, and the type of disease they cause. The smallpox virus remains the most notable member of the family.[verification needed]
See also
References
- ↑ 1.0 1.1 1.2 "Poxviridae ~ ViralZone". viralzone.expasy.org. Archived from the original on 11 November 2023. Retrieved 17 January 2024.
- ↑ 2.0 2.1 "Virus Taxonomy: 2019 Release". talk.ictvonline.org. International Committee on Taxonomy of Viruses. Archived from the original on 20 March 2020. Retrieved 9 May 2020.
- ↑ "Pathogenic Molluscum Contagiosum Virus Sequenced". Antiviral Agents Bulletin: 196–7. August 1996. Archived from the original on 4 March 2023. Retrieved 16 July 2006.
- ↑ "Poxvirus Diseases | Pox Viruses | CDC". www.cdc.gov. 27 February 2023. Archived from the original on 30 September 2023. Retrieved 17 January 2024.
- ↑ Bohelay, G; Duong, T-A (1 May 2019). "[Human poxvirus infections]". Annales de dermatologie et de venereologie. 146 (5): 387–398. doi:10.1016/j.annder.2019.03.001. ISSN 0151-9638. Archived from the original on 25 January 2024. Retrieved 24 January 2024.
- ↑ Efridi, Wajahat; Lappin, Sarah L. (2024). "Poxviruses". StatPearls. StatPearls Publishing. Archived from the original on 27 May 2022. Retrieved 27 January 2024.
- ↑ International Committee on Taxonomy of Viruses (15 June 2004). "ICTVdb Descriptions: 58. Poxviridae". Archived from the original on 4 March 2023. Retrieved 26 February 2005.
- ↑ How Big is a ... ? Archived 29 May 2021 at the Wayback Machine at Cells Alive! Archived 4 March 2023 at the Wayback Machine. Retrieved 2005-02-26.
- ↑ Gubser, C; Hué, S; Kellam, P; Smith, GL (2004). "Poxvirus genomes: a phylogenetic analysis". J Gen Virol. 85 (1): 105–117. doi:10.1099/vir.0.19565-0. PMID 14718625.
- ↑ Roychoudhury, S; Pan, A; Mukherjee, D (2011). "Genus specific evolution of codon usage and nucleotide compositional traits of poxviruses". Virus Genes. 42 (2): 189–199. doi:10.1007/s11262-010-0568-2. PMID 21369827. S2CID 21779605.
- ↑ Deng, Zhaobin; Xia, Xuyang; Deng, Yiqi; Zhao, Mingde; Gu, Congwei; Geng, Yi; Wang, Jun; Yang, Qian; He, Manli; Xiao, Qihai; Xiao, Wudian; He, Lvqin; Liang, Sicheng; Xu, Heng; Lü, Muhan; Yu, Zehui (5 May 2022). "ANI analysis of poxvirus genomes reveals its potential application to viral species rank demarcation". Virus Evolution. 8 (1). doi:10.1093/ve/veac031. Archived from the original on 7 May 2022. Retrieved 21 January 2024.
- ↑ Tu, Shin-Lin; Upton, Chris (2019). "Bioinformatics for Analysis of Poxvirus Genomes". Methods in Molecular Biology (Clifton, N.J.). 2023: 29–62. doi:10.1007/978-1-4939-9593-6_2. ISSN 1940-6029. Archived from the original on 1 September 2022. Retrieved 18 January 2024.
- ↑ Yu, Zehui; Zhang, Wenjie; Fu, Huancheng; Zou, Xiaoxia; Zhao, Mingde; Liang, Sicheng; Gu, Congwei; Yang, Qian; He, Manli; Xiao, Qihai; Xiao, Wudian; He, Lvqin; Lü, Muhan (28 September 2021). "Genomic analysis of Poxviridae and exploring qualified gene sequences for phylogenetics". Computational and Structural Biotechnology Journal. 19: 5479–5486. doi:10.1016/j.csbj.2021.09.031. ISSN 2001-0370. Archived from the original on 20 January 2024. Retrieved 19 January 2024.
- ↑ "Orthopoxvirus replication ~ ViralZone". viralzone.expasy.org. Archived from the original on 4 March 2023. Retrieved 26 June 2022.
- ↑ 15.0 15.1 Moss, Bernard (1 September 2013). "Poxvirus DNA replication". Cold Spring Harbor Perspectives in Biology. 5 (9): a010199. doi:10.1101/cshperspect.a010199. ISSN 1943-0264. Archived from the original on 23 October 2023. Retrieved 23 January 2024.
- ↑ Schramm, Birgit; Locker, Jacomine Krijnse (October 2005). "Cytoplasmic Organization of POXvirus DNA Replication". Traffic. 6 (10): 839–846. doi:10.1111/j.1600-0854.2005.00324.x. ISSN 1398-9219. Archived from the original on 28 January 2024. Retrieved 28 January 2024.
- ↑ Buller, R M; Palumbo, G J (March 1991). "Poxvirus pathogenesis". Microbiological Reviews. 55 (1): 80–122. doi:10.1128/mr.55.1.80-122.1991. ISSN 0146-0749. Archived from the original on 11 June 2022. Retrieved 3 February 2024.
- ↑ National Center for Infectious Diseases, Centers for Disease Control and Prevention, 1600 Clifton Rd., Atlanta, GA 30333, USA. "DNA-dependent RNA polymerase rpo35 (Vaccinia virus)". National Center for Biotechnology Information (NCBI), NIH, Bethesda, MD, USA. Archived from the original on 4 March 2023. Retrieved 30 January 2023.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ↑ Racaniello, Vincent (4 March 2014). "Pithovirus: Bigger than Pandoravirus with a smaller genome". Virology Blog. Archived from the original on 25 December 2022. Retrieved 4 March 2014.
- ↑ Mutsafi, Y; Zauberman, N; Sabanay, I; Minsky, A (30 March 2010). "Vaccinia-like cytoplasmic replication of the giant Mimivirus". Proceedings of the National Academy of Sciences USA. 107 (13): 5978–82. Bibcode:2010PNAS..107.5978M. doi:10.1073/pnas.0912737107. PMC 2851855. PMID 20231474..
- ↑ Verardi, Paulo H.; Titong, Allison; Hagen, Caitlin J. (July 2012). "A vaccinia virus renaissance: New vaccine and immunotherapeutic uses after smallpox eradication". Human Vaccines & Immunotherapeutics. 8 (7): 961–970. doi:10.4161/hv.21080. ISSN 2164-5515. Archived from the original on 26 October 2022. Retrieved 17 January 2024.
- ↑ James, William D.; Elston, Dirk M.; Treat, James R.; Rosenbach, Misha A. (18 January 2019). Andrews' Diseases of the Skin: Clinical Dermatology. Elsevier Health Sciences. p. 387. ISBN 978-0-323-55188-5. Archived from the original on 10 January 2023. Retrieved 27 January 2024.
- ↑ Tolonen, N.; Doglio, L.; Schleich, S.; Krijnse Locker, J. (July 2001). "Vaccinia virus DNA replication occurs in endoplasmic reticulum-enclosed cytoplasmic mini-nuclei". Molecular Biology of the Cell. 12 (7): 2031–2046. doi:10.1091/mbc.12.7.2031. ISSN 1059-1524. Archived from the original on 1 September 2023. Retrieved 22 January 2024.
- ↑ Yen, Judy; Golan, Ron; Rubins, Kathleen (13 April 2009). "Vaccinia Virus Infection & Temporal Analysis of Virus Gene Expression: Part 3". Journal of Visualized Experiments : JoVE (26): 1170. doi:10.3791/1170. ISSN 1940-087X. Archived from the original on 28 January 2024. Retrieved 25 January 2024.
- ↑ Yang, Zhilong; Maruri-Avidal, Liliana; Sisler, Jerry; Stuart, Carey A.; Moss, Bernard (1 December 2013). "Cascade regulation of vaccinia virus gene expression is modulated by multistage promoters". Virology. 447 (1): 213–220. doi:10.1016/j.virol.2013.09.007. ISSN 0042-6822.
- ↑ D.K, Dubey R. C. & Maheshwari (2023). A Textbook of Microbiology:. S. Chand Publishing. p. 492. ISBN 978-93-5501-527-3. Archived from the original on 10 February 2024. Retrieved 30 January 2024.
- ↑ McInnes, Colin J.; Damon, Inger K.; Smith, Geoffrey L.; McFadden, Grant; Isaacs, Stuart N.; Roper, Rachel L.; Evans, David H.; Damaso, Clarissa R.; Carulei, Olivia; Wise, Lyn M.; Lefkowitz, Elliot J. (May 2023). "ICTV Virus Taxonomy Profile: Poxviridae 2023". The Journal of General Virology. 104 (5). doi:10.1099/jgv.0.001849. ISSN 1465-2099. Archived from the original on 18 May 2023. Retrieved 22 January 2024.
- ↑ "Family: Poxviridae | ICTV". ictv.global. Archived from the original on 25 September 2023. Retrieved 28 January 2024.
- ↑ Afonso PP, Silva PM, Schnellrath LC, Jesus DM, Hu J, Yang Y, Renne R, Attias M, Condit RC, Moussatché N, Damaso CR (2012) Biological characterization and next-generation genome sequencing of the unclassified Cotia virus SPAn232 (Poxviridae). J Virol
- ↑ "Taxonomy History [Cotia virus] - Taxonomy - ICTV". talk.ictvonline.org. Archived from the original on 4 March 2023. Retrieved 30 January 2023.
- ↑ Hora, AS; Taniwaki, SA; Martins, NB; Pinto, NNR; Schlemper, AE; Santos, ALQ; Szabó, MPJ; Brandão, PE (April 2021). "Genomic Analysis of Novel Poxvirus Brazilian Porcupinepox Virus, Brazil, 2019". Emerging Infectious Diseases. 27 (4): 1177–1180. doi:10.3201/eid2704.203818. PMC 8007330. PMID 33754985.
- ↑ Smithson C, Meyer H, Gigante CM, Gao J, Zhao H, Batra D, Damon I, Upton C, Li Y (2017) Two novel poxviruses with unusual genome rearrangements: NY_014 and Murmansk. Virus Genes
- ↑ "Taxonomy History [Yokapox virus] - Taxonomy - ICTV". talk.ictvonline.org. Archived from the original on 4 March 2023. Retrieved 30 January 2023.
- ↑ Bahar, MW; Graham, SC; Stuart, DI; Grimes, JM (2011). "Insights into the evolution of a complex virus from the crystal structure of vaccinia virus D13". Structure. 19 (7): 1011–1020. doi:10.1016/j.str.2011.03.023. PMC 3136756. PMID 21742267.
- ↑ Prangishvili, D; Garrett, RA (2004). "Exceptionally diverse morphotypes and genomes of crenarchaeal hyperthermophilic viruses" (PDF). Biochem Soc Trans (Submitted manuscript). 32 (2): 204–208. doi:10.1042/bst0320204. PMID 15046572. Archived (PDF) from the original on 15 April 2023. Retrieved 30 January 2023.
- ↑ Babkin IV, Shchelkunov SN (2006) The time scale in poxvirus evolution. Mol Biol (Mosk) 40(1):20-24
- ↑ 37.0 37.1 37.2 Babkin, IV; Babkina, IN (2011). "Molecular dating in the evolution of vertebrate poxviruses". Intervirology. 54 (5): 253–260. doi:10.1159/000320964. PMID 21228539.
- ↑ 38.0 38.1 38.2 38.3 Hughes, AL; Irausquin, S; Friedman, R (2010). "The evolutionary biology of poxviruses". Infect Genet Evol. 10 (1): 50–59. doi:10.1016/j.meegid.2009.10.001. PMC 2818276. PMID 19833230.
- ↑ Gjessing, MC; Yutin, N; Tengs, T; Senkevich, T; Koonin, E; Rønning, HP; Alarcon, M; Ylving, S; Lie, KI; Saure, B; Tran, L; Moss, B; Dale, OB (2015). "Salmon Gill Poxvirus, the Deepest Representative of the Chordopoxvirinae". J Virol. 89 (18): 9348–9867. doi:10.1128/JVI.01174-15. PMC 4542343. PMID 26136578.
- ↑ Wibbelt, Gudrun; Tausch, Simon H.; Dabrowski, Piotr W.; Kershaw, Olivia; Nitsche, Andreas; Schrick, Livia (2017). "Berlin Squirrelpox Virus, a New Poxvirus in Red Squirrels, Berlin, Germany". Emerging Infectious Diseases. 23 (10): 1726–1729. doi:10.3201/eid2310.171008. PMC 5621524. PMID 28930029.(for systematic see figure 2)
- ↑ Esposito, JJ; Sammons, SA; Frace, AM; Osborne, JD; Olsen-Rasmussen, M; Zhang, M; Govil, D; Damon, IK; et al. (August 2006). "Genome sequence diversity and clues to the evolution of variola (smallpox) virus". Science (Submitted manuscript). 313 (5788): 807–812. Bibcode:2006Sci...313..807E. doi:10.1126/science.1125134. PMID 16873609. S2CID 39823899. Archived from the original on 8 October 2022. Retrieved 30 January 2023.
- ↑ Li, Y; Carroll, DS; Gardner, SN; Walsh, MC; Vitalis, EA; Damon, IK (2007). "On the origin of smallpox: correlating variola phylogenics with historical smallpox records". Proc Natl Acad Sci USA. 104 (40): 15787–15792. Bibcode:2007PNAS..10415787L. doi:10.1073/pnas.0609268104. PMC 2000395. PMID 17901212.
- ↑ "Signs and Symptoms | Smallpox | CDC". www.cdc.gov. 15 February 2019. Archived from the original on 7 June 2020. Retrieved 21 January 2024.
- ↑ "Smallpox". Armed Forces Institute of Pathology: Department of Infectious and Parasitic Diseases. Archived from the original on 9 October 2007. Retrieved 28 October 2008.
- ↑ "Diagnosis & Evaluation | Smallpox | CDC". www.cdc.gov. 15 February 2019. Archived from the original on 9 December 2017. Retrieved 21 January 2024.
- ↑ Atkinson W, Hamborsky J, McIntyre L, Wolfe S, eds. (2005). "Smallpox" (PDF). Epidemiology and Prevention of Vaccine-Preventable Diseases (The Pink Book) (9th ed.). Washington DC: Public Health Foundation. pp. 281–306. Archived from the original (PDF) on 6 March 2010.
- ↑ "Vaccine Overview" (PDF). Smallpox Fact Sheet. Archived from the original (PDF) on 2 January 2008. Retrieved 2 January 2008.
- ↑ Office of the Commissioner. "Press Announcements – FDA approves the first drug with an indication for treatment of smallpox". www.fda.gov. Archived from the original on 23 April 2019. Retrieved 28 July 2018.
- ↑ Flight C (17 February 2011). "Smallpox: Eradicating the Scourge". BBC History. Archived from the original on 14 February 2009. Retrieved 28 July 2015.
- ↑ Grimes W (25 November 2010). "Frank Fenner Dies at 95". The New York Times. Archived from the original on 9 March 2014. Retrieved 27 November 2010.
- ↑ Preston R (12 July 1999). "A reporter at large: Demon in the Freezer". The New Yorker. Archived from the original on 7 September 2014. Retrieved 3 January 2008.
- ↑ "Smallpox". WHO Factsheet. Archived from the original on 21 September 2007.
- ↑ Fenner F (2006). Nature, Nurture and Chance: The Lives of Frank and Charles Fenner. Canberra, ACT 0200: Australian National University Press. ISBN 978-1-920942-62-5.
{{cite book}}: CS1 maint: location (link) - ↑ 54.0 54.1 Riedel, Stefan (January 2005). "Edward Jenner and the history of smallpox and vaccination". Proceedings (Baylor University. Medical Center). Baylor University Medical Center. 18 (1): 21–25. doi:10.1080/08998280.2005.11928028. PMC 1200696. PMID 16200144.
- ↑ 55.0 55.1 Baxby, Derrick (2009) [2004]. "Jenner, Edward (1749–1823)". Oxford Dictionary of National Biography (online ed.). Oxford University Press. doi:10.1093/ref:odnb/14749. (Subscription or UK public library membership required.)
- ↑ Hopkins, Donald R. (2002) [1983]. The greatest killer: smallpox in history, with a new introduction. University of Chicago Press. p. 15.
By special permission of the late President Anwar el Sadat, I was allowed to examine the front upper half of Ramses V's unwrapped mummy in the Cairo Museum in 1979. …Inspection of the mummy revealed a rash of elevated "pustules," each about two to four millimeters in diameter, …(An attempt to prove that this rash was caused by smallpox by electron-microscopic examination of tiny pieces of tissue that had fallen on the shroud was unsuccessful. I was not permitted to excise one of the postules.) …The appearance of the larger pustules and the apparent distribution of the rash are similar to smallpox rashes I have seen in more recent victims
- ↑ Date of Ramses V's death derived from the Encyclopedia of Ancient Egypt, Margaret Bunson (New York: Facts On File, 2002) ISBN 0816045631 p.337.
- ↑ "History of Smallpox | Smallpox | CDC". www.cdc.gov. 21 February 2021. Archived from the original on 14 June 2020. Retrieved 21 January 2024.
- ↑ "WHO commemorates the 40th anniversary of smallpox eradication". www.who.int. Archived from the original on 14 January 2024. Retrieved 2 February 2024.
- ↑ Henderson, D. A.; Inglesby, Thomas V.; Bartlett, John G.; Ascher, Michael S.; Eitzen, Edward; Jahrling, Peter B.; Hauer, Jerome; Layton, Marcelle; McDade, Joseph; Osterholm, Michael T.; O'Toole, Tara; Parker, Gerald; Perl, Trish; Russell, Philip K.; Tonat, Kevin; For The Working Group On Civilian Biodefense (1999). "Smallpox as a Biological Weapon: Medical and Public Health Management". JAMA: The Journal of the American Medical Association. 281 (22): 2127–37. doi:10.1001/jama.281.22.2127. PMID 10367824.
- ↑ Chan, Winnie M.; McFadden, Grant (1 September 2014). "Oncolytic Poxviruses". Annual Review of Virology. 1 (1): 119–141. doi:10.1146/annurev-virology-031413-085442. ISSN 2327-056X. PMC 4380149. PMID 25839047.
- ↑ Evgin, Laura; Vähä-Koskela, Markus; Rintoul, Julia; Falls, Theresa; Le Boeuf, Fabrice; Barrett, John W.; Bell, John C.; Stanford, Marianne M. (May 2010). "Potent oncolytic activity of raccoonpox virus in the absence of natural pathogenicity". Molecular Therapy. 18 (5): 896–902. doi:10.1038/mt.2010.14. ISSN 1525-0024. PMC 2890119. PMID 20160706.
- ↑ Suryawanshi, Yogesh R.; Zhang, Tiantian; Razi, Farzad; Essani, Karim (July 2020). "Tanapoxvirus: From discovery towards oncolytic immunovirotherapy". Journal of Cancer Research and Therapeutics. 16 (4): 708–712. doi:10.4103/jcrt.JCRT_157_18. ISSN 1998-4138. PMID 32930107.
Further reading
- Electron micrographs of Orthopoxvirus and Parapoxvirus Genera, including the smallpox virus, have been collected by the International Committee on Taxonomy of Viruses in their Poxviridae picture gallery Archived 18 February 2009 at the Wayback Machine.
- Buller, R. Mark L.; Palumbo, Gregory J. (1991). "Poxvirus Pathogenesis". Microbiology and Molecular Biology Reviews. 55 (1): 80–122. doi:10.1128/mr.55.1.80-122.1991. PMC 372802. PMID 1851533.
- Evans, David Hugh (9 August 2022). "Poxvirus Recombination". Pathogens (Basel, Switzerland). 11 (8): 896. doi:10.3390/pathogens11080896. ISSN 2076-0817. Archived from the original on 15 November 2022. Retrieved 25 January 2024.
External links
- NCBI Taxonomy Page Archived 16 May 2023 at the Wayback Machine.
- Poxviridae at the Viral Bioinformatics Resource Center Archived 5 January 2012 at the Wayback Machine.
- Viralzone: Poxviridae Archived 4 March 2023 at the Wayback Machine
- ICTV Archived 10 July 2015 at the Wayback Machine
- Virus Pathogen Database and Analysis Resource (ViPR): Poxviridae
- Webarchive template wayback links
- CS1 maint: multiple names: authors list
- CS1 maint: location
- Wikipedia articles incorporating a citation from the ODNB
- Use dmy dates from June 2021
- Articles with invalid date parameter in template
- Articles with 'species' microformats
- Articles with hatnote templates targeting a nonexistent page
- All pages needing factual verification
- Wikipedia articles needing factual verification
- Poxviruses
- Virus families