Pneumococcal infection
| Pneumococcal infection | |
|---|---|
| Other names: Pneumococcosis | |
 | |
| A digitally-colorized, scanning electron microscope (SEM) image of three, round-shaped, Gram-positive, Streptococcus pneumoniae bacteria (lavender), as they were being attacked by an irregularly-shaped white blood cell (WBC) (pink). | |
| Specialty | Infectious diseases |
| Symptoms | Fever, chills, cough, ear pain, headache [1] |
| Causes | Streptococcus pneumoniae[1] |
| Risk factors | Young children, elderly, weakened immune system, no spleen, smokers, pre-existing heart/lung/kidney disease, diabetes, overcrowding[1] |
| Diagnostic method | Tests on blood, sputum, CSF,[1] chest x-ray, lumbar puncture[1] |
| Prevention | Pneumococcal vaccine, respiratory hygiene, good housing, stop smoking, diabetes control, long-term penicillin with people with no spleen[2] |
| Treatment | Antibiotics[1] |
| Medication |
|
| Prognosis | |
| Frequency | Common worldwide[1] |
| Deaths | 1% (sepsis), 5% (meningitis)[1] |
Pneumococcal infection, also known as pneumococcal disease is a vaccine-preventable disease caused by the bacteria Streptococcus pneumoniae.[1] Types include severe disease such as sepsis, and meningitis, pneumonia, and milder infections such as ear infection and sinusitis.[3][4] There is typically a fever associated with other symptoms depending on the part of the body that is infected; cough and breathlessness in pneumonia; ear pain in otitis media, facial pain in sinusitis, tiredness and confusion in sepsis; headache, stiff neck, and photophobia in meningitis.[1][4] Affected babies may be irritable and feed poorly.[1]
S. pneumoniae colonizes the nose and throat of 5–10% of healthy adults and 20–40% of healthy children.[5] Spread occurs from contact with small droplets from an infected person with or without symptoms.[1] Risk of infection is higher in young children, the elderly, people with weakened immune systems, cigarette smokers, and those living in crowded conditions.[1] Having diabetes, heart disease, kidney or lung disease, a cochlear implant, or not having a spleen also increases the risk.[1] Diagnosis is by symptoms and isolating the bacteria from body fluids such as blood, sputum, or cerebrospinal fluid.[1] Blood tests typically show a high white cell count, though in severe disease the white count might drop low.[1] Other findings include raised inflammatory markers.[1] A chest X-ray or lumbar puncture may be required.[1] Other diseases that may appear similar include Haemophilus influenzae infection.[1]
Prevention is by pneumococcal vaccine, and good respiratory hygiene.[2] Avoiding overcrowded living conditions reduces the risk of disease.[1] Long-term penicillin is generally recommended for those with no spleen.[1] For children with sickle cell disease, prevention with penicillin needs to begin before 4 months of age and continued until at least 5-years old in those fully vaccinated.[1] Treatment is with antibiotics; typically a cephalosporin or penicillin, and depending on sensitivities.[1] Higher doses are generally required in meningitis; vancomycin, ceftriaxone, or cefotaxime.[1] Most children with treated mild disease recover.[1] Pneumonia may require admission to hospital.[1] Long term complications may occur following sepsis and meningitis.[1] Death may occur in around 1% of pneumococcal sepsis and 5% of pneumococcal meningitis.[1]
Pneumococcal infection is a significant cause of illness and death in young children and elderly worldwide.[3] In 2008 around 5% of all child deaths under the age of 5 years was due to S. pneumoniae.[1] Worldwide it is the most common cause of childhood bacterial pneumonia.[1] A considerable economic burden results from medical service use for pneumococcal ear infections.[3]
Signs and symptoms

The presentation of pneumococcal infection depends on what has been infected. In the case of lung infection we find:[6]
In the event of meningitis one finds the symptoms/signs to be:[6]
- Fever
- Headache
- Confusion
- Stiff neck
If the affected individual has a blood infection then:[6]
- Low alertness
- Chills
- Fever
Complications
In terms of complications one finds that pneumonia may complicate towards empyema, pericarditis and endobronchial obstruction[6]
Cause
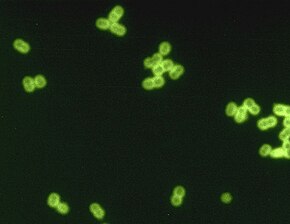
Streptococcus pneumoniae is a Gram-positive, spherical bacteria, alpha-hemolytic (under aerobic conditions) or beta-hemolytic (under anaerobic conditions), aerotolerant anaerobic member of the genus Streptococcus.[7]
They are usually found in pairs (diplococci) and do not form spores and are non motile.[8]
Streptococcus pneumoniae is the main cause of community acquired pneumonia and meningitis in children and the elderly,[9] and of sepsis in those infected with HIV.
The organism also causes many types of pneumococcal infections other than pneumonia. These invasive pneumococcal diseases include bronchitis, rhinitis, acute sinusitis, otitis media, conjunctivitis, meningitis, sepsis, osteomyelitis, septic arthritis, endocarditis, peritonitis, pericarditis, cellulitis, and brain abscess.[10]
Pathogenesis

In terms of S. pneumoniae is normally found in the nose and throat of 5–10% of healthy adults and 20–40% of healthy children.[5]
It can be found in higher amounts, where people are spending a great deal of time in close proximity to each other . It attaches to nasopharyngeal cells through interaction of bacterial surface adhesins. This normal colonization can become infectious if the organisms are carried into areas such as the Eustachian tube or nasal sinuses where it can cause otitis media and sinusitis, respectively; pneumonia occurs if the organisms are inhaled into the lungs and not cleared. The organism's polysaccharide capsule makes it resistant to phagocytosis and if there is no pre-existing anticapsular antibody alveolar macrophages cannot adequately kill the pneumococci. The organism spreads to the blood stream and is carried to the meninges, joint spaces, bones, and peritoneal cavity, and may result in meningitis, or septic arthritis.[11][12][13]
S. pneumoniae has several virulence factors, including the polysaccharide capsule mentioned earlier, that help it evade a host's immune system. It has pneumococcal surface proteins that inhibit complement-mediated opsonization, and it secretes IgA1 protease that will destroy secretory IgA produced by the body and mediates its attachment to respiratory mucosa.[11][14][15]
The risk for developing a pneumococcal infection is increased in individuals with reduced IgG synthesis, impaired phagocytosis and defective bacterial clearance and higher age.[16] In particular, the absence of a functional spleen, through congenital asplenia, surgical removal of the spleen, or sickle-cell disease predisposes one to a more severe course of infection and prevention measures are indicated .[17][18]
People with a compromised immune system, such as those living with HIV, are also at higher risk of pneumococcal disease. In HIV patients with access to treatment, the risk of invasive pneumoccal disease is 0.2–1% per year and has a fatality rate of 8%.[10] There is an association between pneumococcal pneumonia and influenza.[19] Other risk factors include smoking, injection drug use, Hepatitis C, and COPD.[10]
Virulence factors

S. pneumoniae expresses different virulence factors on its cell surface and inside the organism, these virulence factors contribute to some of the clinical manifestations during infection with S. pneumoniae:
- Polysaccharide capsule—prevents phagocytosis by host immune cells by inhibiting C3b opsonization of the bacterial cells[20]
- Pneumolysin (Ply)—a pore-forming protein that can cause lysis of host cells and activate complement[21]
- Autolysin (LytA)—activation of this protein lyses the bacteria releasing its internal contents[22]
- Hydrogen peroxide—causes damage to host cells (can cause apoptosis in neuronal cells during meningitis) and has bactericidal effects against competing bacteria (Haemophilus influenzae, Neisseria meningitidis, Staphylococcus aureus)[23][24]
- Pili—hair-like structures that extend from the surface of many strains of S. pneumoniae. They contribute to colonization of upper respiratory tract and increase the formation of large amounts of TNF by the immune system during sepsis, raising the possibility of septic shock[25]
- Choline binding protein A/Pneumococcal surface protein A (CbpA/PspA)—an adhesin that can interact with carbohydrates on the cell surface of pulmonary epithelial cells and can inhibit complement-mediated opsonization of pneumococci[26]
- Competence for genetic transformation likely plays an important role in nasal colonization fitness and virulence (lung infectivity)[27]
Diagnosis
Depending on the nature of infection an appropriate sample is collected for laboratory identification. Pneumococci are typically gram-positive cocci seen in pairs or chains. When cultured on blood agar plates with added optochin antibiotic disk they show alpha-hemolytic colonies and a clear zone of inhibition around the disk indicating sensitivity to the antibiotic. Pneumococci are also bile soluble. Just like other streptococci they are catalase-negative. A Quellung test can identify specific capsular polysaccharides.[28]
Pneumococcal antigen (cell wall C polysaccharide) may be detected in various body fluids. Older detection kits, based on latex agglutination, added little value above Gram staining and were occasionally false-positive. Better results are achieved with rapid immunochromatography, which has a sensitivity (identifies the cause) of 70–80% and >90% specificity (when positive identifies the actual cause) in pneumococcal infections. The test was initially validated on urine samples but has been applied successfully to other body fluids.[28] Chest X-rays can also be conducted to confirm inflammation though are not specific to the causative agent.[29][13]
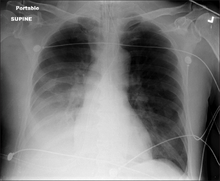
-
Chest X-ray pneumonia
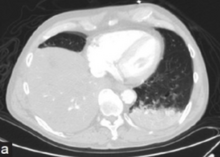
-
CT pneumonia
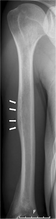
-
X-ray pneumococcal osteomyelitis
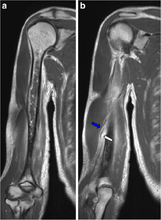
-
MRI pneumococcal osteomyelitis
Prevention
Due to the importance of disease caused by S. pneumoniae several vaccines have been developed to protect against invasive infection.[3]
The World Health Organization recommend routine childhood pneumococcal vaccination;[30] it is incorporated into the childhood immunization schedule in a number of countries including the United Kingdom,[31] United States,[3] and South Africa.[32]
Treatment

Throughout history treatment relied primarily on β-lactam antibiotics, in the later half of the twentieth century nearly all strains of S. pneumoniae were susceptible to penicillin, but more recently there has been an increasing prevalence of penicillin resistance especially in areas of high antibiotic use. A varying proportion of strains may also be resistant to cephalosporins, macrolides, tetracycline, fluoroquinolones. Penicillin-resistant strains are more likely to be resistant to other antibiotics; most isolates remain susceptible to vancomycin.[29][13][33][34]
More advanced beta-lactam antibiotics (cephalosporins) are commonly used in combination with other antibiotics to treat meningitis and community-acquired pneumonia. In adults recently developed fluoroquinolones such as levofloxacin and moxifloxacin are often used to provide empiric coverage for patients with pneumonia, but in parts of the world where these medications are used to treat tuberculosis, resistance has been described.[35]
Susceptibility testing should be routine with empiric antibiotic treatment guided by resistance patterns in the community in which the organism was acquired. There is currently debate as to how relevant the results of susceptibility testing are to clinical outcome.[36][37] There is slight clinical evidence that penicillins may act synergistically with macrolides to improve outcomes.[38]
Resistant Pneumococci strains are called penicillin-resistant Pneumococci (PRP),[39] penicillin-resistant Streptococcus pneumoniae (PRSP),[40] Streptococcus pneumoniae penicillin resistant (SPPR),[41] or drug-resistant Strepotococcus pneumomoniae (DRSP).[42]
Epidemiology
In terms of epidemiology of the Pneumococcal infection we find it has had the following effects:
- Pneumococcal pneumonia represents 15%–50% of all episodes of community-acquired pneumonia.[43]
- It represents 30–50% of all cases of acute otitis media, and a significant proportion of bloodstream infections and bacterial meningitis.[43]
- As estimated by WHO in 2005 it killed about 1.6 million children every year worldwide with 0.7–1 million of them being under the age of five. The majority of these deaths were in developing countries.[44]
History


In 1881, the organism, known later in 1886 as the pneumococcus[45] for its role as a cause of pneumonia, was first isolated simultaneously and independently by the U.S. Army physician George Sternberg[46] and the French chemist Louis Pasteur.[47]
In the 19th century it was demonstrated that immunization of rabbits with killed pneumococci protected them against subsequent challenge with viable pneumococci. Serum from immunized rabbits or from humans who had recovered from pneumococcal pneumonia also conferred protection. In the 20th century, the efficacy of immunization was demonstrated in South African miners.[48][49]
It was discovered that the pneumococcus's capsule made it resistant to phagocytosis, and in the 1920s it was shown that an antibody specific for capsular polysaccharide aided the killing of S. pneumoniae. In 1936, a pneumococcal capsular polysaccharide vaccine was used to abort an epidemic of pneumococcal pneumonia. In the 1940s, experiments on capsular transformation by pneumococci first identified DNA as the material that carries genetic information.[50]
In the 1900's it was recognized that different serovars of pneumococci exist , since then over ninety serovars have been discovered each with a unique polysaccharide capsule that can be identified by the quellung reaction. Because some of these serovars cause disease more commonly than others it is possible to provide reasonable protection by immunizing with less than 90 serovars; current vaccines contain up to 23 serovars (i.e., it is "23-valent").The serovars are numbered according to two systems: the American system, which numbers them in the order in which they were discovered, and the Danish system, which groups them according to antigenic similarities.[48][51][52]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 1.32 1.33 1.34 1.35 Pratt, R. Douglas (2022). "5. Pneumococcal disease: infections caused by Streptococcus pneumonia". In Jong, Elaine C.; Stevens, Dennis L. (eds.). Netter's Infectious Diseases (2nd ed.). Philadelphia: Elsevier. pp. 20–23. ISBN 978-0-323-71159-3. Archived from the original on 2023-10-20. Retrieved 2023-09-29.
- ↑ 2.0 2.1 "Pneumococcal Disease: Prevention | CDC". www.cdc.gov. 21 September 2023. Archived from the original on 13 April 2014. Retrieved 29 September 2023.
- ↑ 3.0 3.1 3.2 3.3 3.4 Dagan, Ron; Ben-Shimol, Shalom (2021). "21. Pneumococcal vaccine". In Vesikari, Timo; Damme, Pierre Van (eds.). Pediatric Vaccines and Vaccinations: A European Textbook (Second ed.). Switzerland: Springer. pp. 223–248. ISBN 978-3-030-77172-0. Archived from the original on 2023-10-20. Retrieved 2023-09-30.
- ↑ 4.0 4.1 "Types of Pneumococcal Disease | CDC". www.cdc.gov. 2023-03-13. Archived from the original on 2023-06-08. Retrieved 2023-09-29.
- ↑ 5.0 5.1 Ryan KJ; Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 0-8385-8529-9.
- ↑ 6.0 6.1 6.2 6.3 "Symptoms and Complications of Pneumococcal Disease | CDC". www.cdc.gov. 28 July 2022. Archived from the original on 8 May 2019. Retrieved 2 February 2023.
- ↑ "Pinkbook: Pneumococcal Disease | CDC". www.cdc.gov. 21 September 2022. Archived from the original on 1 September 2022. Retrieved 2 February 2023.
- ↑ "Streptococcus pneumoniae". microbewiki.kenyon.edu. Archived from the original on 2019-03-27. Retrieved 2017-10-24.
- ↑ van de Beek, Diederik; de Gans, Jan; Tunkel, Allan R.; Wijdicks, Eelco F.M. (5 January 2006). "Community-Acquired Bacterial Meningitis in Adults". New England Journal of Medicine. 354 (1): 44–53. doi:10.1056/NEJMra052116. ISSN 0028-4793. PMID 16394301.
- ↑ 10.0 10.1 10.2 Siemieniuk, Reed A.C.; Gregson, Dan B.; Gill, M. John (Nov 2011). "The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study". BMC Infectious Diseases. 11: 314. doi:10.1186/1471-2334-11-314. PMC 3226630. PMID 22078162.
- ↑ 11.0 11.1 McDaniel, Larry S.; Swiatlo, Edwin (March 2004). "Pneumococcal Disease: Pathogenesis, Treatment, and Prevention". Infectious Diseases in Clinical Practice. 12 (2): 93. doi:10.1097/01.idc.0000121024.62151.c9. ISSN 1056-9103. Archived from the original on 30 June 2016. Retrieved 4 February 2023.
- ↑ Gillespie, S. H.; Balakrishnan, I. (December 2000). "Pathogenesis of pneumococcal infection". Journal of Medical Microbiology. 49 (12): 1057–1067. doi:10.1099/0022-1317-49-12-1057. ISSN 0022-2615. Archived from the original on 5 February 2023. Retrieved 5 February 2023.
- ↑ 13.0 13.1 13.2 Dion, Christopher F.; Ashurst, John V. (2022). "Streptococcus Pneumoniae". StatPearls. StatPearls Publishing. Archived from the original on 22 October 2022. Retrieved 6 February 2023.
- ↑ Mitchell, A. M.; Mitchell, T. J. (1 May 2010). "Streptococcus pneumoniae: virulence factors and variation". Clinical Microbiology and Infection. 16 (5): 411–418. doi:10.1111/j.1469-0691.2010.03183.x. ISSN 1198-743X. Archived from the original on 8 February 2023. Retrieved 8 February 2023.
- ↑ Mistry, Dippica; Stockley, Robert A. (January 2006). "IgA1 protease". The International Journal of Biochemistry & Cell Biology. 38 (8): 1244–1248. doi:10.1016/j.biocel.2005.10.005. Archived from the original on 10 February 2023. Retrieved 10 February 2023.
- ↑ Ludwig, Heinz; Boccadoro, Mario; Moreau, Philippe; San-Miguel, Jesus; Cavo, Michele; Pawlyn, Charlotte; Zweegman, Sonja; Facon, Thierry; Driessen, Christoph; Hajek, Roman; Dimopoulos, Melitios A.; Gay, Francesca; Avet-Loiseau, Hervé; Terpos, Evangelos; Zojer, Niklas; Mohty, Mohamad; Mateos, Maria-Victoria; Einsele, Hermann; Delforge, Michel; Caers, Jo; Weisel, Katja; Jackson, Graham; Garderet, Laurent; Engelhardt, Monika; van de Donk, Niels; Leleu, Xavier; Goldschmidt, Hartmut; Beksac, Meral; Nijhof, Inger; Abildgaard, Niels; Bringhen, Sara; Sonneveld, Pieter (January 2021). "Recommendations for vaccination in multiple myeloma: a consensus of the European Myeloma Network". Leukemia. 35 (1): 31–44. doi:10.1038/s41375-020-01016-0. ISSN 1476-5551. PMC 7787974. PMID 32814840. Archived from the original on 6 January 2023. Retrieved 11 February 2023.
 This article incorporates text by Heinz Ludwig, Mario Boccadoro, Philippe Moreau, Jesus San-Miguel, Michele Cavo, Charlotte Pawlyn, Sonja Zweegman, Thierry Facon, Christoph Driessen, Roman Hajek, Melitios A. Dimopoulos, Francesca Gay, Hervé Avet-Loiseau, Evangelos Terpos, Niklas Zojer, Mohamad Mohty, Maria-Victoria Mateos, Hermann Einsele, Michel Delforge, Jo Caers, Katja Weisel, Graham Jackson, Laurent Garderet, Monika Engelhardt, and Pieter Sonneveld available under the CC BY 4.0 license.
This article incorporates text by Heinz Ludwig, Mario Boccadoro, Philippe Moreau, Jesus San-Miguel, Michele Cavo, Charlotte Pawlyn, Sonja Zweegman, Thierry Facon, Christoph Driessen, Roman Hajek, Melitios A. Dimopoulos, Francesca Gay, Hervé Avet-Loiseau, Evangelos Terpos, Niklas Zojer, Mohamad Mohty, Maria-Victoria Mateos, Hermann Einsele, Michel Delforge, Jo Caers, Katja Weisel, Graham Jackson, Laurent Garderet, Monika Engelhardt, and Pieter Sonneveld available under the CC BY 4.0 license.
- ↑ "Pneumococcal Disease: Risk Factors and How It Spreads | CDC". www.cdc.gov. 28 July 2022. Archived from the original on 1 April 2019. Retrieved 5 February 2023.
- ↑ "Pneumococcal Disease Risk Factors: Information for Clinicians | CDC". www.cdc.gov. 28 February 2022. Archived from the original on 14 December 2022. Retrieved 7 February 2023.
- ↑ Walter ND, Taylor TH, Shay DK, et al. (2010). "Influenza Circulation and the Burden of Invasive Pneumococcal Pneumonia during a Non‐pandemic Period in the United States". Clin Infect Dis. 50 (2): 175–183. doi:10.1086/649208. PMID 20014948.
- ↑ Paton, James C.; Trappetti, Claudia (March 2019). "Streptococcus pneumoniae Capsular Polysaccharide". Microbiology Spectrum. 7 (2). doi:10.1128/microbiolspec.GPP3-0019-2018. ISSN 2165-0497. Archived from the original on 7 July 2022. Retrieved 8 February 2023.
- ↑ Nishimoto, Andrew T.; Rosch, Jason W.; Tuomanen, Elaine I. (2020). "Pneumolysin: Pathogenesis and Therapeutic Target". Frontiers in Microbiology. 11: 1543. Archived from the original on 28 July 2022. Retrieved 9 February 2023.
- ↑ Jedrzejas, M. J. (June 2001). "Pneumococcal virulence factors: structure and function". Microbiology and molecular biology reviews: MMBR. 65 (2): 187–207, first page, table of contents. doi:10.1128/MMBR.65.2.187-207.2001. ISSN 1092-2172. Archived from the original on 27 February 2022. Retrieved 9 February 2023.
- ↑ Pericone, Christopher D.; Overweg, Karin; Hermans, Peter W. M.; Weiser, Jeffrey N. (2000). "Inhibitory and Bactericidal Effects of Hydrogen Peroxide Production by Streptococcus pneumoniae on Other Inhabitants of the Upper Respiratory Tract". Infect Immun. 68 (7): 3990–3997. doi:10.1128/IAI.68.7.3990-3997.2000. PMC 101678. PMID 10858213.
- ↑ Regev-Yochay G, Trzcinski K, Thompson CM, Malley R, Lipsitch M (2006). "Interference between Streptococcus pneumoniae and Staphylococcus aureus: In vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae". J Bacteriol. 188 (13): 4996–5001. doi:10.1128/JB.00317-06. PMC 1482988. PMID 16788209.
- ↑ Barocchi M, Ries J, Zogaj X, Hemsley C, Albiger B, Kanth A, Dahlberg S, Fernebro J, Moschioni M, Masignani V, Hultenby K, Taddei A, Beiter K, Wartha F, von Euler A, Covacci A, Holden D, Normark S, Rappuoli R, Henriques-Normark B (2006). "A pneumococcal pilus influences virulence and host inflammatory responses". Proc Natl Acad Sci USA. 103 (8): 2857–2862. Bibcode:2006PNAS..103.2857B. doi:10.1073/pnas.0511017103. PMC 1368962. PMID 16481624.
- ↑ Sempere, Julio; Llamosí, Mirella; del Río Menéndez, Idoia; López Ruiz, Beatriz; Domenech, Mirian; González-Camacho, Fernando (20 February 2021). "Pneumococcal Choline-Binding Proteins Involved in Virulence as Vaccine Candidates". Vaccines. 9 (2): 181. doi:10.3390/vaccines9020181. Archived from the original on 9 February 2023. Retrieved 9 February 2023.
- ↑ Li G, Liang Z, Wang X, Yang Y, Shao Z, Li M, Ma Y, Qu F, Morrison DA, Zhang JR (2016). "Addiction of Hypertransformable Pneumococcal Isolates to Natural Transformation for In Vivo Fitness and Virulence". Infect. Immun. 84 (6): 1887–901. doi:10.1128/IAI.00097-16. PMC 4907133. PMID 27068094.
- ↑ 28.0 28.1 Werno AM, Murdoch DR (March 2008). "Medical microbiology: laboratory diagnosis of invasive pneumococcal disease". Clin. Infect. Dis. 46 (6): 926–32. doi:10.1086/528798. PMID 18260752.
- ↑ 29.0 29.1 "Diagnosis and Treatment of Pneumococcal Disease | CDC". www.cdc.gov. 28 July 2022. Archived from the original on 10 December 2022. Retrieved 3 February 2023.
- ↑ "Pneumococcal vaccines WHO position paper—2012" (PDF). Wkly Epidemiol Rec. 87 (14): 129–44. Apr 6, 2012. PMID 24340399. Archived (PDF) from the original on March 4, 2016. Retrieved September 14, 2021.
- ↑ "Children to be given new vaccine". BBC News. 8 February 2006. Archived from the original on 30 May 2006. Retrieved 14 September 2021.
- ↑ "Critical decline in pneumococcal disease and antibiotic resistance in South Africa". NICD. Archived from the original on 23 July 2015. Retrieved 20 July 2015.
- ↑ Moumne, Olivia; Duff, Patrick (December 2019). "Treatment and Prevention of Pneumococcal Infection". Clinical Obstetrics and Gynecology. 62 (4): 781–789. doi:10.1097/GRF.0000000000000451. ISSN 1532-5520. Archived from the original on 2023-02-06. Retrieved 2023-02-06.
- ↑ "Drug resistant STREPTOCOCCUS PNEUMONIAE" (PDF). CDC. Archived (PDF) from the original on 29 August 2022. Retrieved 15 February 2023.
- ↑ Group For Enteric; Von Gottberg, A.; Klugman, K. P.; Cohen, C.; Wolter, N.; De Gouveia, L.; Du Plessis, M.; Mpembe, R.; Quan, V.; Whitelaw, A.; Hoffmann, R.; Govender, N.; Meiring, S.; Smith, A. M.; Schrag, S. (2008). "Emergence of levofloxacin-non-susceptible Streptococcus pneumoniae and treatment for multidrug-resistant tuberculosis in children in South Africa: a cohort observational surveillance study". The Lancet. 371 (9618): 1108–1113. doi:10.1016/S0140-6736(08)60350-5. PMID 18359074. S2CID 205950081.
- ↑ Peterson LR (2006). "Penicillins for treatment of pneumococcal pneumonia: does in vitro resistance really matter?". Clin Infect Dis. 42 (2): 224–33. doi:10.1086/497594. PMID 16355333.
- ↑ Tleyjeh IM, Tlaygeh HM, Hejal R, Montori VM, Baddour LM (2006). "The impact of penicillin resistance on short-term mortality in hospitalized adults with pneumococcal pneumonia: a systematic review and meta-analysis". Clin Infect Dis. 42 (6): 788–97. doi:10.1086/500140. PMID 16477555.
- ↑ Martínez JA, Horcajada JP, Almela M, et al. (2003). "Addition of a Macrolide to a β-Lactam based empirical antibiotic regimen is associated with lower in-hospital mortality for patients with bacteremic pneumococcal pneumonia". Clin Infect Dis. 36 (4): 389–395. doi:10.1086/367541. PMID 12567294.
- ↑ Nilsson, P; Laurell, MH (2001). "Carriage of penicillin-resistant Streptococcus pneumoniae by children in day-care centers during an intervention program in Malmo, Sweden". The Pediatric Infectious Disease Journal. 20 (12): 1144–9. doi:10.1097/00006454-200112000-00010. PMID 11740321.
- ↑ Block, SL; Harrison, CJ; Hedrick, JA; Tyler, RD; Smith, RA; Keegan, E; Chartrand, SA (1995). "Penicillin-resistant Streptococcus pneumoniae in acute otitis media: risk factors, susceptibility patterns and antimicrobial management". The Pediatric Infectious Disease Journal. 14 (9): 751–9. doi:10.1097/00006454-199509000-00005. PMID 8559623.
- ↑ Koiuszko, S; Bialucha, A; Gospodarek, E (2007). "[The drug susceptibility of penicillin-resistant Streptococcus pneumoniae]". Medycyna Doswiadczalna I Mikrobiologia. 59 (4): 293–300. PMID 18416121.
- ↑ "Drug Resistance". cdc.gov. 2019-02-13. Archived from the original on 2019-01-12. Retrieved 17 February 2019.
- ↑ 43.0 43.1 Verma R, Khanna P (2012) Pneumococcal conjugate vaccine: A newer vaccine available in India. Hum Vaccin Immunother 8(9)
- ↑ WHO (2007). "Pneumococcal conjugate vaccine for childhood immunization—WHO position paper" (PDF). Wkly Epidemiol Rec. Geneva: World Health Organization. 82 (12): 93–104. PMID 17380597. Archived (PDF) from the original on 2016-03-04. Retrieved 2021-09-14.
- ↑ Plotkin, Stanley; Orenstein, W; Offit, PA (September 22, 2012). Vaccines. Elsevier – Saunders. p. 542. ISBN 978-1455700905. Archived from the original on February 2, 2023. Retrieved July 2, 2015.
- ↑ Sternberg, George Miller (30 April 1881). "A fatal form of septicaemia in the rabbit produced by the subcutaneous injection of human saliva. An experimental research". Bulletin of the National Board of Health..
- ↑ Pasteur, Louis (1881). "Sur une maladie nouvelle provoquée par la salive d'un enfant mort de rage". Comptes Rendus de l'Académie des Sciences de Paris. 92: 159..
- ↑ 48.0 48.1 Geno, K. Aaron; Gilbert, Gwendolyn L.; Song, Joon Young; Skovsted, Ian C.; Klugman, Keith P.; Jones, Christopher; Konradsen, Helle B.; Nahm, Moon H. (July 2015). "Pneumococcal Capsules and Their Types: Past, Present, and Future". Clinical Microbiology Reviews. 28 (3): 871–899. doi:10.1128/CMR.00024-15. Archived from the original on 23 December 2022. Retrieved 7 February 2023.
- ↑ Grabenstein, J.D.; Klugman, K.P. (October 2012). "A century of pneumococcal vaccination research in humans". Clinical Microbiology and Infection. 18: 15–24. doi:10.1111/j.1469-0691.2012.03943.x. Archived from the original on 30 April 2019. Retrieved 12 February 2023.
- ↑ Avery OT, Macleod CM, McCarty M (1944). "Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types". J. Exp. Med. 79 (2): 137–58. doi:10.1084/jem.79.2.137. PMC 2135445. PMID 19871359.
- ↑ Daniels, Calvin C.; Rogers, P. David; Shelton, Chasity M. (1 January 2016). "A Review of Pneumococcal Vaccines: Current Polysaccharide Vaccine Recommendations and Future Protein Antigens". The Journal of Pediatric Pharmacology and Therapeutics. 21 (1): 27–35. doi:10.5863/1551-6776-21.1.27. Archived from the original on 7 December 2022. Retrieved 10 February 2023.
- ↑ Flaherty, Dennis (25 June 2014). Immunology for Pharmacy - E-Book. Elsevier Health Sciences. p. 202. ISBN 978-0-323-29111-8. Archived from the original on 12 February 2023. Retrieved 11 February 2023.
External links
| Classification |
|---|