Lipodystrophy
| Lipodystrophie | |
|---|---|
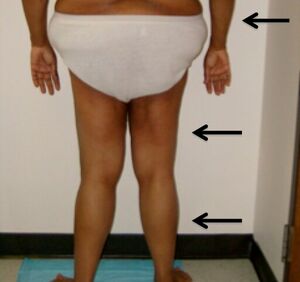 | |
| Individual is shown with arrows indicating lipodystrophy of the forearms, thighs, and legs. | |
| Specialty | Endocrinology |
Lipodystrophy is a group of genetic or acquired disorders in which the body is unable to produce and maintain healthy fat tissue.[1][2] It is characterized by abnormal or degenerative conditions of the body's adipose tissue. A more specific term, lipoatrophy ("lipo" is Greek for "fat", and "dystrophy" is Greek for "abnormal or degenerative condition"), is used when describing the loss of fat from one area (usually the face). This condition is also characterized by a lack of circulating leptin which may lead to osteosclerosis. The absence of fat tissue is associated with insulin resistance, hypertriglyceridemia, non-alcoholic fatty liver disease (NAFLD) and metabolic syndrome.[3][4]
Types
Lipodystrophy can be divided into the following types:[5]: 495–7
- Congenital lipodystrophy syndromes
- Acquired lipodystrophy syndromes
- Acquired partial lipodystrophy (Barraquer-Simons syndrome)
- Acquired generalized lipodystrophy
- Centrifugal abdominal lipodystrophy (Lipodystrophia centrifugalis abdominalis infantilis)
- Lipoatrophia annularis (Ferreira-Marques lipoatrophia)
- Localized lipodystrophy
- HIV-associated lipodystrophy
Risk factors
Antiretroviras
Lipodystrophy can be a possible side effect of antiretroviral drugs. Other lipodystrophies manifest as lipid redistribution, with excess, or lack of, fat in various regions of the body. These include, but are not limited to, having sunken cheeks and/or "humps" on the back or back of the neck (also referred to as buffalo hump)[7] which also exhibits due to excess cortisol. Lipoatrophy is most commonly seen in patients treated with thymidine analogue nucleoside reverse transcriptase inhibitors [8] like zidovudine (AZT) and stavudine (d4T).[9]
Insulin injections
A lipodystrophy can be a lump or small dent in the skin that forms when a person performs injections repeatedly in the same spot. These types of lipodystrophies are harmless and can be avoided by changing (rotating) the locations of injections. For those with diabetes, using purified insulins may also help.One of the side-effects of lipodystrophy is the rejection of the injected medication, the slowing down of the absorption of the medication, or trauma that can cause bleeding that, in turn, will reject the medication. In any of these scenarios, the dosage of the medication, such as insulin for diabetics, becomes impossible to gauge correctly and the treatment of the disease for which the medication is administered is impaired, thereby allowing the medical condition to worsen.In some cases, rotation of the injection sites may not be enough to prevent lipodystrophy.
Pathogenesis
Due to an insufficient capacity of subcutaneous adipose tissue to store fat, fat is deposited in non-adipose tissue (lipotoxicity), leading to insulin resistance.[10] Patients display hypertriglyceridemia, severe fatty liver disease and little or no adipose tissue.[11] Average patient lifespan is approximately 30 years before death, with liver failure being the usual cause of death.[11] In contrast to the high levels seen in non-alcoholic fatty liver disease associated with obesity, leptin levels are very low in lipodystropy.[10]
Diagnosis
The diagnosis is a clinical diagnosis, established by an experienced endocrinologist. A genetic confirmation may be possible depending on the subtype. In up to ~40% of partial lipodystrophy patients, a causative gene has not been identified.[3] Using a skinfold caliper to measure skinfold thickness in various parts of the body or a total body composition scan using Dual-energy X-ray Absorptiometry may help identify the subtype.[4][12] Dual-energy X-ray Absorptiometry may be useful by providing both regional %fat measurements, and direct visualization of fat distribution by means of a "fat shadow".[13]
Treatment
Leptin replacement therapy with human recombinant leptin metreleptin has been shown to be an effective therapy to alleviate the metabolic complications associated with lipodystrophy, and has been approved by the FDA for the treatment of generalized lipodystrophy syndromes.[14] In Europe based on EMA, metreleptin should be used in addition to diet to treat lipodystrophy, where patients have loss of fatty tissue under the skin and build-up of fat elsewhere in the body such as in the liver and muscles. The medicine is used in: adults and children above the age of two years with generalised lipodystrophy (Berardinelli-Seip syndrome and Lawrence syndrome) and in adults and children above the age of 12 years with partial lipodystrophy (including Barraquer-Simons syndrome), when standard treatments have failed.[15]
Volanesorsen is an Apo-CIII inhibitor[16][17] that is currently being investigated as a potential therapeutic to reduce hypertriglycerides in Familial Partial Lipodystrophy patients in the BROADEN study.[18]
Epidemiology
Congenital lipodystrophy (due to inherited genetic defect) is estimated to be extremely rare, possibly affecting only one per million persons.[10] Acquired lipodystrophy is much more common, especially affecting persons with HIV infection.[10]
Society and culture
Lipodystrophy United is an American organization founded and run by lipodystrophy patients to support each other and raise awareness about lipodystrophy syndromes.[19]
Lipodystrophy UK is a dedicated UK charity set up to support people affected by Lipodystrophy.[20]
March 31 is observed as the World Lipodystrophy Day.[21][22]
See also
References
- ↑ Phan J, Reue K (January 2005). "Lipin, a lipodystrophy and obesity gene". Cell Metabolism. 1 (1): 73–83. doi:10.1016/j.cmet.2004.12.002. PMID 16054046.
- ↑ "UCLA/VA Researchers discover fat gene". Archived from the original on 2018-10-06. Retrieved 2017-06-15.
- ↑ 3.0 3.1 Brown RJ, Araujo-Vilar D, Cheung PT, Dunger D, Garg A, Jack M, Mungai L, Oral EA, Patni N, Rother KI, von Schnurbein J, Sorkina E, Stanley T, Vigouroux C, Wabitsch M, Williams R, Yorifuji T (December 2016). "The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline". The Journal of Clinical Endocrinology and Metabolism. 101 (12): 4500–4511. doi:10.1210/jc.2016-2466. PMC 5155679. PMID 27710244.
- ↑ 4.0 4.1 Ajluni N, Meral R, Neidert AH, Brady GF, Buras E, McKenna B, DiPaola F, Chenevert TL, Horowitz JF, Buggs-Saxton C, Rupani AR, Thomas PE, Tayeh MK, Innis JW, Omary MB, Conjeevaram H, Oral EA (May 2017). "Spectrum of disease associated with partial lipodystrophy: lessons from a trial cohort". Clinical Endocrinology. 86 (5): 698–707. doi:10.1111/cen.13311. PMC 5395301. PMID 28199729.
- ↑ James, William D.; Berger, Timothy G.; et al. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 978-0-7216-2921-6.
- ↑ Torrelo A, Patel S, Colmenero I, Gurbindo D, Lendínez F, Hernández A, López-Robledillo JC, Dadban A, Requena L, Paller AS (March 2010). "Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome". Journal of the American Academy of Dermatology. 62 (3): 489–95. doi:10.1016/j.jaad.2009.04.046. PMID 20159315.
- ↑ Physical and Biochemical Changes in HIV Disease Archived 2012-03-05 at the Wayback Machine Eric S. Daar, M.D. MedicineNet, Accessed 22 September 2007
- ↑ Carr A, Workman C, Smith DE, Hoy J, Hudson J, Doong N, Martin A, Amin J, Freund J, Law M, Cooper DA (July 2002). "Abacavir substitution for nucleoside analogs in patients with HIV lipoatrophy: a randomized trial". JAMA. 288 (2): 207–15. doi:10.1001/jama.288.2.207. PMID 12095385.
- ↑ John M, McKinnon EJ, James IR, Nolan DA, Herrmann SE, Moore CB, White AJ, Mallal SA (May 2003). "Randomized, controlled, 48-week study of switching stavudine and/or protease inhibitors to combivir/abacavir to prevent or reverse lipoatrophy in HIV-infected patients". Journal of Acquired Immune Deficiency Syndromes. 33 (1): 29–33. doi:10.1097/00126334-200305010-00005. PMID 12792352. S2CID 22845453.
- ↑ 10.0 10.1 10.2 10.3 Polyzos SA, Perakakis N, Mantzoros CS (2019). "Fatty liver in lipodystrophy: A review with a focus on therapeutic perspectives of adiponectin and/or leptin replacement". Metabolism: Clinical and Experimental. 96: 66–82. doi:10.1016/j.metabol.2019.05.001. PMID 31071311.
- ↑ 11.0 11.1 Bruder-Nascimento T, Kress TC, Belin de Chantemele EJ (2019). "Recent advances in understanding lipodystrophy: a focus on lipodystrophy-associated cardiovascular disease and potential effects of leptin therapy on cardiovascular function". F1000Research. 8: F1000 Faculty Rev-1756. doi:10.12688/f1000research.20150.1. PMC 6798323. PMID 31656583.
- ↑ Guillín-Amarelle C, Sánchez-Iglesias S, Castro-Pais A, Rodriguez-Cañete L, Ordóñez-Mayán L, Pazos M, González-Méndez B, Rodríguez-García S, Casanueva FF, Fernández-Marmiesse A, Araújo-Vilar D (November 2016). "Type 1 familial partial lipodystrophy: understanding the Köbberling syndrome". Endocrine. 54 (2): 411–421. doi:10.1007/s12020-016-1002-x. PMID 27473102. S2CID 19689303.
- ↑ Meral R, Ryan BJ, Malandrino N, Jalal A, Neidert AH, Muniyappa R, Akıncı B, Horowitz JF, Brown RJ, Oral EA (October 2018). ""Fat Shadows" From DXA for the Qualitative Assessment of Lipodystrophy: When a Picture Is Worth a Thousand Numbers". Diabetes Care. 41 (10): 2255–2258. doi:10.2337/dc18-0978. PMC 6150431. PMID 30237235.
- ↑ Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A (February 2002). "Leptin-replacement therapy for lipodystrophy". The New England Journal of Medicine. 346 (8): 570–8. doi:10.1056/NEJMoa012437. PMID 11856796.
- ↑ "Myalepta | European Medicines Agency". www.ema.europa.eu. Archived from the original on 2021-04-10. Retrieved 2019-01-08.
- ↑ Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, Geary RS, Baker BF, Graham MJ, Crooke RM, Witztum JL (December 2014). "Targeting APOC3 in the familial chylomicronemia syndrome". The New England Journal of Medicine. 371 (23): 2200–6. doi:10.1056/NEJMoa1400284. PMID 25470695.
- ↑ Gaudet D, Alexander VJ, Baker BF, Brisson D, Tremblay K, Singleton W, Geary RS, Hughes SG, Viney NJ, Graham MJ, Crooke RM, Witztum JL, Brunzell JD, Kastelein JJ (July 2015). "Antisense Inhibition of Apolipoprotein C-III in Patients with Hypertriglyceridemia". The New England Journal of Medicine. 373 (5): 438–47. doi:10.1056/NEJMoa1400283. PMID 26222559. S2CID 205096489. Archived from the original on 2023-07-01. Retrieved 2023-05-06.
- ↑ "The BROADEN Study: A Study of Volanesorsen (Formerly ISIS-APOCIIIRx) in Patients With Familial Partial Lipodystrophy - Full Text View - ClinicalTrials.gov". Archived from the original on 2018-04-01. Retrieved 2018-03-31.
- ↑ "Welcome to Lipodystrophy United". www.lipodystrophyunited.org. Archived from the original on 2019-10-03. Retrieved 2018-03-31.
- ↑ "Lipodystrophy UK". www.lipodystrophyuk.org. Archived from the original on 2020-02-29. Retrieved 2020-02-29.
- ↑ Inc., Ionis Pharmaceuticals. "Akcea Therapeutics Supports World Lipodystrophy Day". www.prnewswire.com. Archived from the original on 2018-04-01. Retrieved 2018-03-31.
- ↑ "Aegerion Pharmaceuticals Observes World Lipodystrophy Day – PM360". www.pm360online.com. Archived from the original on 2018-04-01. Retrieved 2018-03-31.
External links
| Classification | |
|---|---|
| External resources |