Norcodeine
Jump to navigation
Jump to search
 | |
| Clinical data | |
|---|---|
| Dependence liability | High |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.718 |
| Chemical and physical data | |
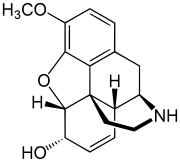
| Formula | C17H19NO3 |
| Molar mass | 285.343 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Norcodeine is an opiate analogue that is the N-demethylated derivative of codeine. It has relatively little opioid activity in its own right,[2] but is formed as a metabolite of codeine following ingestion.[3]
Norcodeine is a Schedule I Narcotic controlled substance in the US with the ACSCN of 9309 and zero annual manufacturing quota. The salts in use are the acetate (free base conversion ratio 0.826), hydroiodide (0.662), hydrochloride (0.759), nitrate (0.819), platinichloride (0.582), and sulphate (0.744).[4]
See also
References
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-03.
- ^ Fraser HF, Isbell H, Vanhorn GD (June 1960). "Human pharmacology and addiction liability of norcodeine". The Journal of Pharmacology and Experimental Therapeutics. 129: 172–7. PMID 13824628.
- ^ Posey BL, Kimble SN (1984). "High-performance liquid chromatographic study of codeine, norcodeine, and morphine as indicators of codeine ingestion". Journal of Analytical Toxicology. 8 (2): 68–74. doi:10.1093/jat/8.2.68. PMID 6716978.
- ^ "Quotas - 2014". DEA Diversion Control Division.
Categories:
- CS1 Brazilian Portuguese-language sources (pt-br)
- Articles with short description
- Short description is different from Wikidata
- Drugs not assigned an ATC code
- Articles with changed ChemSpider identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- 4,5-Epoxymorphinans
- Phenol ethers
- Secondary alcohols
- Opiates
- Opioid metabolites
- All stub articles
- Nervous system drug stubs