Sorafenib
 | |
 | |
| Names | |
|---|---|
| Trade names | Nexavar, others |
| Other names | Nexavar Sorafenib tosylate |
| |
| Clinical data | |
| Drug class | Protein kinase inhibitor[1] |
| Main uses | Renal cell carcinoma, hepatocellular carcinoma, thyroid cancer[2][1] |
| Side effects | Diarrhea, rash, hair loss, infection, rash, tiredness[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Defined daily dose | 400 mg BID[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607051 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 38–49% |
| Protein binding | 99.5% |
| Metabolism | Liver oxidation and glucuronidation (CYP3A4 & UGT1A9-mediated) |
| Elimination half-life | 25–48 hours |
| Excretion | Faeces (77%) and urine (19%) |
| Chemical and physical data | |
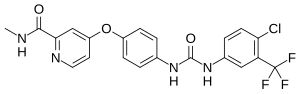
| Formula | C21H16ClF3N4O3 |
| Molar mass | 464.83 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Sorafenib, sold under the brand name Nexavar, is a medication used to treat renal cell carcinoma, hepatocellular carcinoma, and certain types of thyroid cancer.[2][1] It is taken by mouth.[1]
Common side effects include diarrhea, rash, hair loss, infection, rash, and tiredness.[1] Other side effects may include a heart attack, gastrointestinal perforation, liver problems, bleeding, and high blood pressure.[1] Use in pregnancy or breastfeeding may harm the baby.[4] It is a protein kinase inhibitor.[1]
Sorafenib was approved for medical use in the United States in 2005 and Europe in 2006.[2][1] In the United Kingdom 4 weeks of treatment costs the NHS about £3,600 as of 2021.[3] This amount in the United States costs about 20,200 USD.[5]
Medical uses
Sorafenib is indicated as a treatment for advanced renal cell carcinoma (RCC), unresectable hepatocellular carcinomas (HCC) and thyroid cancer.[6][7][8][9]
Kidney cancer
Clinical trial results, published January 2007, showed that, compared with placebo, treatment with sorafenib prolongs progression-free survival in patients with advanced clear cell renal cell carcinoma in whom previous therapy has failed. The median progression-free survival was 5.5 months in the sorafenib group and 2.8 months in the placebo group (hazard ratio for disease progression in the sorafenib group, 0.44; 95% confidence interval [CI], 0.35 to 0.55; P<0.01).[10]
In Australia this is one of two TGA-labelled indications for sorafenib, although it is not listed on the Pharmaceutical Benefits Scheme for this indication.[9][11]
Liver cancer
At ASCO 2007, results from the SHARP trial[12] were presented, which showed efficacy of sorafenib in hepatocellular carcinoma. The primary endpoint was median overall survival, which showed a 44% improvement in patients who received sorafenib compared to placebo (hazard ratio 0.69; 95% CI, 0.55 to 0.87; p=0.0001). Both median survival and time to progression showed 3-month improvements; however, there was no significant difference in median time to symptomatic progression (p=0.77). There was no difference in quality of life measures, possibly attributable to toxicity of sorafenib or symptoms related to underlying progression of liver disease. Of note, this trial only included patients with Child-Pugh Class A (i.e. mildest) cirrhosis.[12] Because of this trial Sorafenib obtained FDA approval for the treatment of advanced hepatocellular carcinoma in November 2007.[13]
In a randomized, double-blind, phase II trial combining sorafenib with doxorubicin, the median time to progression was not significantly delayed compared with doxorubicin alone in patients with advanced hepatocellular carcinoma. Median durations of overall survival and progression-free survival were significantly longer in patients receiving sorafenib plus doxorubicin than in those receiving doxorubicin alone.[13]
A prospective single-centre phase II study which included the patients with unresectable hepatocellular carcinoma (HCC)concluding that the combination of sorafenib and DEB-TACE in patients with unresectable HCC is well tolerated and safe, with most toxicities related to sorafenib.[14]
In Australia this is the only indication for which sorafenib is listed on the PBS and hence the only Government-subsidised indication for sorafenib.[11] Along with renal cell carcinoma, hepatocellular carcinoma is one of the TGA-labelled indications for sorafenib.[9]
Thyroid cancer
On November 22, 2013, sorafenib was approved by the FDA for the treatment of locally recurrent or metastatic, progressive differentiated thyroid carcinoma (DTC) refractory to radioactive iodine treatment.[15]
The Phase 3 DECISION trial showed significant improvement in progression-free survival but not in overall survival. However, as is known, the side effects were very frequent, specially hand and foot skin reaction.[16]
Dosage
It is generally taken at a dose of 400 mg twice per day.[3]
Side effects
By frequency
Very common (>10% frequency)
- Lymphopenia
- Hypophosphataemia[Note 1]
- Haemorrhage[Note 2]
- Hypertension[Note 3]
- Diarrhea
- Rash
- Alopecia (hair loss; occurs in roughly 30% of patients receiving sorafenib)
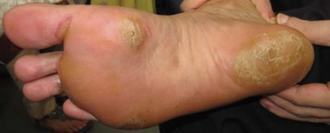
- Hand-foot syndrome
- Pruritus (itchiness)
- Erythema
- Increased amylase
- Increased lipase
- Fatigue
- Pain[Note 4]
- Nausea
- Vomiting[Note 5][17]
Common (1-10% frequency)
- Leucopenia[Note 6]
- Neutropoenia[Note 7]
- Anaemia[Note 8]
- Thrombocytopenia[Note 9]
- Anorexia (weight loss)
- Hypocalcaemia[Note 10]
- Hypokalaemia[Note 11]
- Depression
- Peripheral sensory neuropathy
- Tinnitus[Note 12]
- Congestive heart failure
- Myocardial infarction[Note 13]
- Myocardial ischaemia[Note 14]
- Hoarseness
- Constipation
- Stomatitis[Note 15]
- Dyspepsia[Note 16]
- Dysphagia[Note 17]
- Dry skin
- Exfoliative dermatitis
- Acne
- Skin desquamation
- Arthralgia[Note 18]
- Myalgia[Note 19]
- Kidney failure[Note 20]
- Proteinuria[Note 21]
- Erectile dysfunction
- Asthenia (weakness)
- Fever
- Influenza-like illness
- Transient increase in transaminase
Uncommon (0.1-1% frequency)
- Folliculitis
- Infection
- Hypersensitivity reactions[Note 22]
- Hypothyroidism[Note 23]
- Hyperthyroidism[Note 24]
- Hyponatraemia[Note 25]
- Dehydration
- Reversible posterior leukoencephalopathy
- Hypertensive crisis
- Rhinorrhoea[Note 26]
- Interstitial lung disease-like events[Note 27]
- Gastro-oesophageal reflux disease (GORD)
- Pancreatitis[Note 28]
- Gastritis[Note 29]
- Gastrointestinal perforations[Note 30]
- Increase in bilirubin leading, potentially, to jaundice[Note 31]
- Cholecystitis[Note 32]
- Cholangitis[Note 33]
- Eczema
- Erythema multiforme[Note 34]
- Keratoacanthoma[Note 35]
- Squamous cell carcinoma
- Gynaecomastia (swelling of the breast tissue in men)
- Transient increase in blood alkaline phosphatase
- INR abnormal
- Prothrombin level abnormal
- bulbous skin reaction[18]
Rare (0.01-0.1% frequency)
Mechanism of action
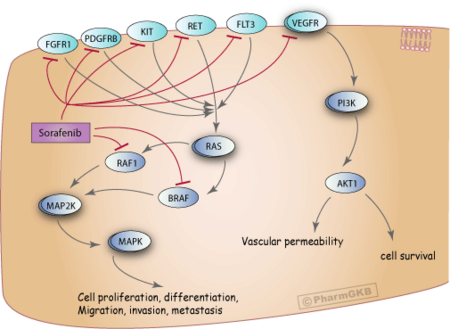
Sorafenib is a protein kinase inhibitor with activity against many protein kinases, including VEGFR, PDGFR and RAF kinases.[20][13]
Of the RAF kinases, Sorafenib is more selective for c-Raf than B-RAF.[21]
Sorafenib treatment induces autophagy,[22] which may suppress tumor growth. Based on its 1,3-disubstituted urea structure, Sorafenib is also a potent soluble epoxide hydrolase inhibitor and this activity likely reduces the severity of its adverse effects.[23]
History
Renal cancer
Sorafenib was approved by the U.S. Food and Drug Administration (FDA) in December 2005,[24] and received European Commission marketing authorization in July 2006,[25] both for use in the treatment of advanced renal cancer.
Liver cancer
The European Commission granted marketing authorization to the drug for the treatment of patients with hepatocellular carcinoma(HCC), the most common form of liver cancer, in October 2007,[26] and FDA approval for this indication followed in November 2007.[27]
In November 2009, the UK's National Institute of Clinical Excellence declined to approve the drug for use within the NHS in England, Wales and Northern Ireland, stating that its effectiveness (increasing survival in primary liver cancer by 6 months) did not justify its high price, at up to £3000 per patient per month.[28] In Scotland the drug had already been refused authorization by the Scottish Medicines Consortium for use within NHS Scotland, for the same reason.[28]
In March 2012, the Indian Patent Office granted a domestic company, Natco Pharma, a license to manufacture generic Sorafenib, bringing its price down by 97%. Bayer sells a month's supply, 120 tablets, of Nexavar for₹280,000 (US$3,500). Natco Pharma will sell 120 tablets for ₹8,800 (US$110), while still paying a 6% royalty to Bayer. The royalty was later raised to 7% on appeal by Bayer.[29][30][31] Under the Patents Act, 1970 and the World Trade Organisation TRIPS Agreement, the government can issue a compulsory license when a drug is not available at an affordable price.[32]
Research
Lung
In some kinds of lung cancer (with squamous-cell histology) sorafenib administered in addition to paclitaxel and carboplatin may be detrimental to patients.[33]
Ovarian cancer
Sorafenib has been studied as maintenance therapy after ovarian cancer treatment and in combination with chemotherapy for recurrent ovarian cancer but did not show results that led to approval of the drug for these indications.[34]
Brain
There is a phase I/II study at the Mayo Clinic[35] of sorafenib and CCI-779 (temsirolimus) for recurrent glioblastoma.
Desmoid tumor
A study performed in 2011 showed that sorafenib is active against aggressive fibromatosis. This study is being used as justification for using Sorafenib as an initial course of treatment in some patients with aggressive fibromatosis.[36]
A phase 3 clinical trial is under way testing the effectiveness of Sorafenib to treat desmoid tumors (also known as aggressive fibromatosis), after positive results in the first two trial stages. Dosage is typically half of that applied for malignant cancers (400 mg vs 800 mg). NCI are sponsoring this trial.[37][38]
Nexavar controversy
In January 2014, Bayer's CEO Marijn Dekkers allegedly stated that Nexavar was developed for "Western Patients Who Can Afford it, not for Indians". However, Dekkers actually never said this. In fact, his words were misquoted and the context was omitted. A kidney cancer patient would pay $96,000 (£58,000) for a year's course of the Bayer-made drug, whereas the cost of the Indian version of the generic drug would be around $2,800 (£1,700).>[39]
Notes
- ↑ Low blood phosphate levels
- ↑ Bleeding; including serious bleeds such as intracranial and intrapulmonary bleeds
- ↑ High blood pressure
- ↑ Including abdominal pain, headache, tumour pain, etc.
- ↑ Considered a low (~10-30%) risk chemotherapeutic agent for causing emesis)
- ↑ Low level of white blood cells in the blood
- ↑ Low level of neutrophils in the blood
- ↑ Low level of red blood cells in the blood
- ↑ Low level of plasma cells in the blood
- ↑ Low blood calcium
- ↑ Low blood potassium
- ↑ Hearing ringing in the ears
- ↑ Heart attack
- ↑ Lack of blood supply for the heart muscle
- ↑ Mouth swelling, also dry mouth and glossodynia
- ↑ Indigestion
- ↑ Not being able to swallow
- ↑ Sore joints
- ↑ Muscle aches
- ↑ Kidney failure
- ↑ Excreting protein [usually plasma proteins] in the urine. Not dangerous in itself but it is indicative kidney damage
- ↑ Including skin reactions and urticaria (hives)
- ↑ Underactive thyroid
- ↑ Overactive thyroid
- ↑ Low blood sodium
- ↑ Runny nose
- ↑ Pneumonitis, radiation pneumonitis, acute respiratory distress, etc.
- ↑ Swelling of the pancreas
- ↑ Swelling of the stomach
- ↑ Formation of a hole in the gastrointestinal tract, leading to potentially fatal bleeds
- ↑ Yellowing of the skin and eyes due to a failure of the liver to adequately cope with the amount of bilirubin produced by the day-to-day actions of the body
- ↑ Swelling of the gallbladder
- ↑ Swelling of the bile duct
- ↑ 34.0 34.1 34.2 A potentially fatal skin reaction
- ↑ A fairly benign form of skin cancer
- ↑ A potentially fatal abnormality in the electrical activity of the heart
- ↑ Swelling of the skin and mucous membranes
- ↑ A potentially fatal allergic reaction
- ↑ Swelling of the liver
- ↑ The rapid breakdown of muscle tissue leading to the build-up of myoglobin in the blood and resulting in damage to the kidneys
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "Nexavar". Archived from the original on 14 October 2021. Retrieved 14 October 2021.
- ↑ 2.0 2.1 2.2 "SORAfenib Monograph for Professionals". Drugs.com. Archived from the original on 24 January 2021. Retrieved 14 October 2021.
- ↑ 3.0 3.1 3.2 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 1056. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "Sorafenib (Nexavar) Use During Pregnancy". Drugs.com. Archived from the original on 29 November 2020. Retrieved 14 October 2021.
- ↑ "Nexavar Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 26 January 2021. Retrieved 14 October 2021.
- ↑ "Nexavar (sorafenib) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 27 December 2013. Retrieved 26 December 2013.
- ↑ "Nexavar (sorafenib) tablet, film coated [Bayer HealthCare Pharmaceuticals Inc.]". DailyMed. Bayer HealthCare Pharmaceuticals Inc. November 2013. Archived from the original on 26 December 2013. Retrieved 26 December 2013.
- ↑ "Nexavar 200mg film-coated tablets - Summary of Product Characteristics (SPC) - (eMC)". electronic Medicines Compendium. Bayer plc. 27 March 2013. Archived from the original on 27 December 2013. Retrieved 26 December 2013.
- ↑ 9.0 9.1 9.2 "PRODUCT INFORMATION NEXAVAR (sorafenib tosylate)" (PDF). TGA eBusiness Services. Bayer Australia Ltd. 12 December 2012. Archived from the original on 14 January 2016. Retrieved 26 December 2013.
- ↑ Escudier, B; Eisen, T; Stadler, WM; Szczylik, C; Oudard, S; Siebels, M; Negrier, S; Chevreau, C; Solska, E; Desai, AA; Rolland, F; Demkow, T; Hutson, TE; Gore, M; Freeman, S; Schwartz, B; Shan, M; Simantov, R; Bukowski, RM (January 2007). "Sorafenib in advanced clear-cell renal-cell carcinoma". New England Journal of Medicine. 356 (2): 125–34. doi:10.1056/NEJMoa060655. PMID 17215530.
- ↑ 11.0 11.1 "Pharmaceutical Benefits Scheme (PBS) -SORAFENIB". Pharmaceutical Benefits Scheme. Australian Government Department of Health. Archived from the original on 27 December 2013. Retrieved 27 December 2013.
- ↑ 12.0 12.1 Llovet; et al. (2008). "Sorafenib in Advanced Hepatocellular Carcinoma". New England Journal of Medicine. 359 (4): 378–90. CiteSeerX 10.1.1.531.1130. doi:10.1056/NEJMoa0708857. PMID 18650514.
- ↑ 13.0 13.1 13.2 Keating GM, Santoro A (2009). "Sorafenib: a review of its use in advanced hepatocellular carcinoma". Drugs. 69 (2): 223–40. doi:10.2165/00003495-200969020-00006. PMID 19228077.
- ↑ Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF (October 2011). "Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma". J. Clin. Oncol. 29 (30): 3960–7. doi:10.1200/JCO.2011.37.1021. PMC 4829081. PMID 21911714.
- ↑ "FDA Approval for Sorafenib Tosylate". National Cancer Institute. 5 October 2006. Archived from the original on 6 April 2015. Retrieved 18 April 2021.
- ↑ "ASCO: Sorafenib Halts Resistant Thyroid Cancer". www.medpagetoday.com. 4 June 2013. Archived from the original on 23 March 2021. Retrieved 18 April 2021.
- ↑ "Chemotherapy-Induced Nausea and Vomiting Treatment & Management". Medscape Reference. WebMD. 3 July 2012. Archived from the original on 27 December 2013. Retrieved 26 December 2013.
- ↑ Hagopian, Benjamin (August 2010). "Unusually Severe Bullous Skin Reaction to Sorafenib: A Case Report". Journal of Medical Cases. 1 (1): 1–3. doi:10.4021/jmc112e.
- ↑ "Sorafenib Pharmacodynamics". PharmGKB. Retrieved 5 March 2024.
- ↑ Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M (October 2008). "Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling". Mol Cancer Ther. 7 (10): 3129–40. doi:10.1158/1535-7163.MCT-08-0013. PMID 18852116.
- ↑ Smalley KS, Xiao M, Villanueva J, Nguyen TK, Flaherty KT, Letrero R, Van Belle P, Elder DE, Wang Y, Nathanson KL, Herlyn M (January 2009). "CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations". Oncogene. 28 (1): 85–94. doi:10.1038/onc.2008.362. PMC 2898184. PMID 18794803.
- ↑ Zhang Y (Jan 2014). "Screening of kinase inhibitors targeting BRAF for regulating autophagy based on kinase pathways". Mol Med Rep. 9 (1): 83–90. doi:10.3892/mmr.2013.1781. PMID 24213221.
- ↑ Singh, Nalin; Hammock, Bruce (March 30, 2020). "Soluble Epoxide Hydrolase". In Offermanns, Stefan; Rosenthal, Walter (eds.). Encyclopedia of Molecular Pharmacology. Springer, Cham. doi:10.1007/978-3-030-21573-6. ISBN 978-3-030-21573-6.
- ↑ "FDA Approval letter for use of sorafenib in advanced renal cancer" (PDF). Archived (PDF) from the original on 2021-03-30. Retrieved 2021-04-18.
- ↑ European Commission – Enterprise and industry. Nexavar Archived 2008-02-01 at the Wayback Machine. Retrieved April 24, 2007.
- ↑ "Nexavar (Sorafenib) Approved for Hepatocellular Carcinoma in Europe" (Press release). Bayer HealthCare Pharmaceuticals and Onyx Pharmaceuticals. October 30, 2007. Archived from the original on February 6, 2012. Retrieved November 10, 2012.
- ↑ "FDA Approval letter for use of sorafenib in inoperable hepatocellular carcinoma" (PDF). Archived (PDF) from the original on 2021-03-31. Retrieved 2021-04-18.
- ↑ 28.0 28.1 "Liver drug 'too expensive'". BBC News. November 19, 2009. Archived from the original on September 11, 2017. Retrieved November 10, 2012.
- ↑ "Archive copy". Archived from the original on 2017-01-03. Retrieved 2021-04-18.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2012-03-21. Retrieved 2012-04-02.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Seven days: 9–15 March 2012". Nature. 483 (7389): 250–1. 2012. Bibcode:2012Natur.483..250.. doi:10.1038/483250a.
- ↑ "India Patents (Amendment) Act, 2005". WIPO. Archived from the original on 16 January 2013. Retrieved 16 January 2013.
- ↑ "Addition of Sorafenib May Be Detrimental in Some Lung Cancer Patients". login.medscape.com. Archived from the original on 2021-08-29. Retrieved 2021-04-18.
- ↑ Ciccone, Marcia A.; Maoz, Asaf; Casabar, Jennifer K.; Machida, Hiroko; Mabuchi, Seiji; Matsuo, Koji (July 2016). "Clinical outcome of treatment with serine-threonine kinase inhibitors in recurrent epithelial ovarian cancer: a systematic review of literature". Expert Opinion on Investigational Drugs. 25 (7): 781–796. doi:10.1080/13543784.2016.1181748. ISSN 1744-7658. PMC 7534810. PMID 27101098. S2CID 28717797.
- ↑ Clinical trial number NCT00329719 for "Sorafenib and Temsirolimus in Treating Patients With Recurrent Glioblastoma" at ClinicalTrials.gov
- ↑ Gounder, MM; Lefkowitz, RA; Keohan, ML; D'Adamo, DR; Hameed, M; Antonescu, CR; Singer, S; Stout, K; Ahn, L; Maki, RG (Jun 2011). "Activity of Sorafenib against desmoid tumor/deep fibromatosis". Clin Cancer Res. 17 (12): 4082–90. doi:10.1158/1078-0432.CCR-10-3322. PMC 3152981. PMID 21447727.
- ↑ "Sorafenib Tosylate in Treating Patients With Desmoid Tumors or Aggressive Fibromatosis". Clinicaltrials.gov. Archived from the original on 2014-11-13. Retrieved 2021-04-18.
- ↑ Gounder, MM; Lefkowitz, RA; Keohan, ML; D'Adamo, DR; Hameed, M; Antonescu, CR; Singer, S; Stout, K; Ahn, L; Maki, RG (15 June 2011). "Activity of Sorafenib against desmoid tumor/deep fibromatosis". Clinical Cancer Research. 17 (12): 4082–90. doi:10.1158/1078-0432.ccr-10-3322. PMC 3152981. PMID 21447727.
- ↑ "Bloomberg's viral misquote". Archived from the original on 2021-02-10. Retrieved 2021-04-18.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- "Sorafenib". National Cancer Institute. Archived from the original on 2020-10-23. Retrieved 2021-04-18.
- Pages using duplicate arguments in template calls
- CS1 maint: date format
- Webarchive template wayback links
- CS1 maint: archived copy as title
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Bayer brands
- Orphan drugs
- Receptor tyrosine kinase inhibitors
- Chloroarenes
- Trifluoromethyl compounds
- Anilines
- Ureas
- Phenol ethers
- Pyridines
- Carboxamides
- RTT