Neuroendocrinology
Neuroendocrinology is the branch of biology (specifically of physiology) which studies the interaction between the nervous system and the endocrine system; i.e. how the brain regulates the hormonal activity in the body.[1] The nervous and endocrine systems often act together in a process called neuroendocrine integration, to regulate the physiological processes of the human body. Neuroendocrinology arose from the recognition that the brain, especially the hypothalamus, controls secretion of pituitary gland hormones, and has subsequently expanded to investigate numerous interconnections of the endocrine and nervous systems.
The endocrine system consists of numerous glands throughout the body that produce and secrete hormones of diverse chemical structure, including peptides, steroids, and neuroamines. Collectively, hormones regulate many physiological processes. The neuroendocrine system is the mechanism by which the hypothalamus maintains homeostasis, regulating reproduction, metabolism, eating and drinking behaviour, energy utilization, osmolarity and blood pressure.
Neuroendocrine system
Hypothalamus
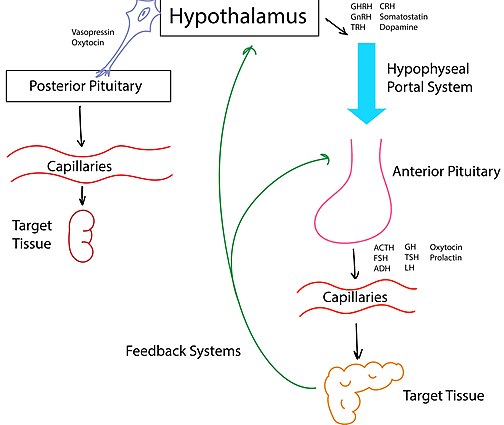
The hypothalamus is commonly known as the relay center of the brain because of its role in integrating inputs from all areas of the brain and producing a specific response. In the neuroendocrine system, the hypothalamus receives electrical signals from different parts of the brain and translates those electrical signals into chemical signals in the form of hormones or releasing factors. These chemicals are then transported to the pituitary gland and from there to the systemic circulation.[2]
Pituitary gland
The pituitary gland is divided into three lobes: the anterior pituitary, the intermediate pituitary lobe, and the posterior pituitary. The hypothalamus controls the anterior pituitary's hormone secretion by sending releasing factors, called tropic hormones, down the hypothalamo-hypophysial portal system.[3] For example, thyrotropin-releasing hormone released by the hypothalamus in to the portal system stimulates the secretion of thyroid-stimulating hormone by the anterior pituitary.[citation needed]
The posterior pituitary is directly innervated by the hypothalamus; the hormones oxytocin and vasopressin are synthesized by neuroendocrine cells in the hypothalamus and stored at the nerve endings in the posterior pituitary. They are secreted directly into systemic circulation by the hypothalamic neurons.[3]
Major neuroendocrine axes
Oxytocin and vasopressin (also called anti-diuretic hormone), the two neurohypophysial hormones of the posterior pituitary gland (the neurohypophysis), are secreted from the nerve endings of magnocellular neurosecretory cells into the systemic circulation. The cell bodies of the oxytocin and vasopressin neurons are in the paraventricular nucleus and supraoptic nucleus of the hypothalamus, respectively,[2] and the electrical activity of these neurons is regulated by afferent synaptic inputs from other brain regions.[4]
By contrast, the hormones of the anterior pituitary gland (the adenohypophysis) are secreted from endocrine cells that, in mammals, are not directly innervated, yet the secretion of these hormones (adrenocorticotrophic hormone, luteinizing hormone, follicle-stimulating hormone, thyroid-stimulating hormone, prolactin, and growth hormone) remains under the control of the hypothalamus. The hypothalamus controls the anterior pituitary gland via releasing factors and release-inhibiting factors; these are substances released by hypothalamic neurons into blood vessels at the base of the brain, at the median eminence.[5] These vessels, the hypothalamo-hypophysial portal vessels, carry the hypothalamic factors to the anterior pituitary, where they bind to specific receptors on the surface of the hormone-producing cells.[3]
For example, the secretion of growth hormone is controlled by two neuroendocrine systems: the growth hormone-releasing hormone (GHRH) neurons and the somatostatin neurons, which stimulate and inhibit GH secretion, respectively.[6] The GHRH neurons are located in the arcuate nucleus of the hypothalamus, whereas the somatostatin cells involved in growth hormone regulation are in the periventricular nucleus. These two neuronal systems project axons to the median eminence, where they release their peptides into portal blood vessels for transport to the anterior pituitary. Growth hormone is secreted in pulses, which arise from alternating episodes of GHRH release and somatostatin release, which may reflect neuronal interactions between the GHRH and somatostatin cells, and negative feedback from growth hormone.[6]
Functions
The neuroendocrine systems control reproduction[7] in all its aspects, from bonding to sexual behaviour. They control spermatogenesis and the ovarian cycle, parturition, lactation, and maternal behaviour. They control the body's response to stress[8] and infection.[9] They regulate the body's metabolism, influencing eating and drinking behaviour, and influence how energy intake is utilised, that is, how fat is metabolised.[10] They influence and regulate mood,[11] body fluid and electrolyte homeostasis,[12] and blood pressure.[13]
The neurons of the neuroendocrine system are large; they are mini factories for producing secretory products; their nerve terminals are large and organised in coherent terminal fields; their output can often be measured easily in the blood; and what these neurons do and what stimuli they respond to are readily open to hypothesis and experiment. Hence, neuroendocrine neurons are good "model systems" for studying general questions, like "how does a neuron regulate the synthesis, packaging, and secretion of its product?" and "how is information encoded in electrical activity?"[citation needed][It appears that this is a primary source observation.]
History
Pioneers
Walter Lee Gaines noted the activity of the pituitary in the lactation of cows in 1915.[14] He also noted that anaesthesia could block lactation and response to the suckling reflex.[15]
Ernst and Berta Scharrer,[16] of the University of Munich the Albert Einstein College of Medicine are credited as co-founders the field of neuroendocrinology with their initial observations and proposals in 1945 concerning neuropeptides.
Geoffrey Harris[17] is considered by many to be the "father" of neuroendocrinology. Harris, the Dr. Lee's Professor of Anatomy at Oxford University, is credited with showing that the anterior pituitary gland of mammals is regulated by hormones secreted by hypothalamic neurons into the hypothalamohypophysial portal circulation. By contrast, the hormones of the posterior pituitary gland are secreted into the systemic circulation directly from the nerve endings of hypothalamic neurons. This seminal work was done in collaboration with Dora Jacobsohn of Lund University.[18]
The first of these factors to be identified are thyrotropin-releasing hormone (TRH) and gonadotropin-releasing hormone (GnRH). TRH is a small peptide that stimulates the secretion of thyroid-stimulating hormone; GnRH (also called luteinizing hormone-releasing hormone) stimulates the secretion of luteinizing hormone and follicle-stimulating hormone.
Roger Guillemin,[19] a medical student of Faculté de Médecine of Lyon, and Andrew W. Schally of Tulane University isolated these factors from the hypothalamus of sheep and pigs, and then identified their structures. Guillemin and Schally were awarded the Nobel Prize in Physiology and Medicine in 1977 for their contributions to understanding "the peptide hormone production of the brain".[citation needed]
In 1952, Andor Szentivanyi, of the University of South Florida, and Geza Filipp wrote the world's first research paper showing how neural control of immunity takes place through the hypothalamus.[20]
Modern scope
Today, neuroendocrinology embraces a wide range of topics that arose directly or indirectly from the core concept of neuroendocrine neurons. Neuroendocrine neurons control the gonads, whose steroids, in turn, influence the brain, as do corticosteroids secreted from the adrenal gland under the influence of adrenocorticotrophic hormone. The study of these feedbacks became the province of neuroendocrinologists. The peptides secreted by hypothalamic neuroendocrine neurons into the blood proved to be released also into the brain, and the central actions often appeared to complement the peripheral actions. So understanding these central actions also became the province of neuroendocrinologists, sometimes even when these peptides cropped up in quite different parts of the brain that appeared to serve functions unrelated to endocrine regulation. Neuroendocrine neurons were discovered in the peripheral nervous system, regulating, for instance, digestion. The cells in the adrenal medulla that release adrenaline and noradrenaline proved to have properties between endocrine cells and neurons, and proved to be outstanding model systems for instance for the study of the molecular mechanisms of exocytosis. And these, too, have become, by extension, neuroendocrine systems.
Neuroendocrine systems have been important to our understanding of many basic principles in neuroscience and physiology, for instance, our understanding of stimulus-secretion coupling.[21] The origins and significance of patterning in neuroendocrine secretion are still dominant themes in neuroendocrinology today.
Neuroendocrinology is also used as an integral part of understanding and treating neurobiological brain disorders. One example is the augmentation of the treatment of mood symptoms with thyroid hormone.[22] Another is the finding of a transthyretin (thyroxine transport) problem in the cerebrospinal fluid of some patients diagnosed with schizophrenia.[23]
Experimental techniques
Since the original experiments by Geoffrey Harris investigating the communication of the hypothalamus with the pituitary gland, much has been learned about the mechanistic details of this interaction. Various experimental techniques have been employed. Early experiments relied heavily on the electrophysiology techniques used by Hodgkin and Huxley. Recent approaches have incorporated various mathematical models to understand previously identified mechanisms and predict systemic response and adaptation under various circumstances.[citation needed]
Electrophysiology
Electrophysiology experiments were used in the early days of neuroendocrinology to identify the physiological happenings in the hypothalamus and the posterior pituitary especially. In 1950, Geoffrey Harris and Barry Cross outlined the oxytocin pathway by studying oxytocin release in response to electrical stimulation.[24] In 1974, Walters and Hatton investigated the effect of water dehydration by electrically stimulating the supraoptic nucleus—the hypothalamic center responsible for the release of vasopressin.[24] Glenn Hatton dedicated his career to studying the physiology of the Neurohypophyseal system, which involved studying the electrical properties of hypothalamic neurons.[24] Doing so enabled investigation into the behavior of these neurons and the resulting physiological effects. Studying the electrical activity of neuroendocrine cells enabled the eventual distinction between central nervous neurons, neuroendocrine neurons, and endocrine cells.[25]
Mathematical Models
Hodgkin-Huxley Model
The Hodgkin–Huxley model translates data about the current of a system at a specific voltage into time-dependent data describing the membrane potential. Experiments using this model typically rely on the same format and assumptions, but vary the differential equations to answer their particular questions. Much has been learned about vasopressin, GnRH, somatotrophs, corticotrophs, and lactotrophic hormones by employing this method.[8]
Integrate-and-Fire Model
The integrate-and-fire model aims for mathematic simplicity in describing biological systems by focusing on, and only on the threshold activity of a neuron. By doing so, the model successfully reduces the complexity of a complicated system; however it ignores the actual mechanisms of action and replaces them with functions that define how the output of a system depends on its input.[8] This model has been used to describe the release of hormones to the posterior pituitary gland, specifically oxytocin and vasopressin.[9]
Functional or Mean Fields Model
The functional or mean fields model relies on the premise "simpler is better".[8] It strives to reduce the complexity of modelling multi-faceted systems by using a single variable to describe an entire population of cells. The alternative would be to use a different set of variables for each population. When attempting to model a system where multiple populations of cells interact, using several sets quickly becomes overcomplicated. This model has been used to describe several systems, especially involving the reproductive cycle (menstrual cycles, luteinizing hormone, prolactin surges).[9] Functional models also exist to represent cortisol secretion, and growth hormone secretion.[9]
See also
References
- ^ "Endocrine system and neuroendocrinology :: DNA Learning Center". www.dnalc.org. Retrieved 2018-05-12.
- ^ a b Watts, Alan G (2015-08-01). "60 Years of Neuroendocrinology: The structure of the neuroendocrine hypothalamus: the neuroanatomical legacy of Geoffrey Harris". Journal of Endocrinology. 226 (2): T25–T39. doi:10.1530/JOE-15-0157. ISSN 0022-0795. PMC 4574488. PMID 25994006.
- ^ a b c Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 10: Neural and Neuroendocrine Control of the Internal Milieu.". In Sydor A, Brown RY (ed.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 246, 248–259. ISBN 9780071481274.
- ^ Honda, Kazumasa; Zhang, William; Tomiyama, Keita (June 2014). "Oxytocin cells in the paraventricular nucleus receive excitatory synaptic inputs from the contralateral paraventricular and supraoptic nuclei in lactating rats". Neuroscience Letters. 572: 44–47. doi:10.1016/j.neulet.2014.04.040. PMID 24792395. S2CID 25107850.
- ^ Knigge, K. M.; Joseph, S. A.; Sladek, J. R.; Notter, M. F.; Morris, M.; Sundberg, D. K.; Holzwarth, M. A.; Hoffman, G. E.; O'Brien, L. (1976-01-01), Bourne, G. H.; Danielli, J. F.; Jeon, K. W. (eds.), Uptake and Transport Activity of the Median Eminence of the Hypothalamus, International Review of Cytology, vol. 45, Academic Press, pp. 383–408, doi:10.1016/s0074-7696(08)60082-0, ISBN 9780123643452, retrieved 2021-11-15
- ^ a b MacGregor, D. J.; Leng, G. (December 2005). "Modelling the Hypothalamic Control of Growth Hormone Secretion". Journal of Neuroendocrinology. 17 (12): 788–803. doi:10.1111/j.1365-2826.2005.01370.x. ISSN 0953-8194. PMID 16280026. S2CID 36712187.
- ^ Blázquez M, Bosma PT, Fraser EJ, Van Look KJ, Trudeau VL (June 1998). "Fish as models for the neuroendocrine regulation of reproduction and growth". Comparative Biochemistry and Physiology. Part C, Pharmacology, Toxicology & Endocrinology. 119 (3): 345–64. doi:10.1016/S0742-8413(98)00023-1. PMID 9827007.
- ^ a b c d Ratka A, Sutanto W, Bloemers M, de Kloet ER (August 1989). "On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation". Neuroendocrinology. 50 (2): 117–23. doi:10.1159/000125210. PMID 2550833.
- ^ a b c d Webster JI, Tonelli L, Sternberg EM (2002). "Neuroendocrine regulation of immunity" (PDF). Annual Review of Immunology. 20: 125–63. doi:10.1146/annurev.immunol.20.082401.104914. PMID 11861600. Archived from the original (PDF) on 2013-12-12.
- ^ McMinn JE, Baskin DG, Schwartz MW (May 2000). "Neuroendocrine mechanisms regulating food intake and body weight". Obesity Reviews. 1 (1): 37–46. doi:10.1046/j.1467-789x.2000.00007.x. PMID 12119644. S2CID 33202919.
- ^ Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, et al. (September 2002). "Neural and behavioral substrates of mood and mood regulation". Biological Psychiatry. 52 (6): 478–502. CiteSeerX 10.1.1.558.6231. doi:10.1016/S0006-3223(02)01458-0. PMID 12361665. S2CID 39992433.
- ^ Antunes-Rodrigues J, de Castro M, Elias LL, Valença MM, McCann SM (January 2004). "Neuroendocrine control of body fluid metabolism". Physiological Reviews. 84 (1): 169–208. doi:10.1152/physrev.00017.2003. PMID 14715914.
- ^ Lenkei Z, Corvol P, Llorens-Cortes C (May 1995). "The angiotensin receptor subtype AT1A predominates in rat forebrain areas involved in blood pressure, body fluid homeostasis and neuroendocrine control". Brain Research. Molecular Brain Research. 30 (1): 53–60. doi:10.1016/0169-328X(94)00272-G. PMID 7609644.
- ^ Medvei, V.C. (2012). A History of Endocrinology. Springer. p. 409.
- ^ Dreifuss, Jean Jacques (1981). "WL Gaines, précurseur du concept de réflexe neuroendocrine". Gesnerus (in French). 38 (3–4): 331–338. doi:10.1163/22977953-0380304004.
- ^ Scharrer E, Scharrer B (1 January 1945). "Neurosecretion". Physiological Reviews. 25 (1): 171–181. doi:10.1152/physrev.1945.25.1.171. ISSN 1522-1210.
- ^ Raisman G (1997). "An urge to explain the incomprehensible: Geoffrey Harris and the discovery of the neural control of the pituitary gland" (PDF). Annual Review of Neuroscience. 20: 533–66. doi:10.1146/annurev.neuro.20.1.533. PMID 9056724. Archived from the original (PDF) on 2007-07-03. Retrieved 2006-02-10.
- ^ Breathnach CS, Moynihan JB (March 2013). "First ladies in laying the foundation of neuroendocrinology" (PDF). Irish Journal of Medical Science. 182 (1): 143–7. doi:10.1007/s11845-012-0830-9. PMID 22581099. S2CID 8177884. Archived from the original (PDF) on 2018-12-24.
- ^ Guillemin R, Schally AV, Lipscomb HS, Andersen RN, Long JM (April 1962). "On the presence in hog hypothalamus of 3-corticotropin releasing factor, alpha- and beta-melanocyte stimulating hormones, adrenocorticotropin, lysine-vasopressin and oxytocin". Endocrinology. 70 (4): 471–7. doi:10.1210/endo-70-4-471. PMID 13902822.
- ^ Berczi I (2010). "Dr Andor Szentivanyi Memorial". University of Manitoba. Archived from the original on 2009-02-10. (Warning: automatic background music)
- ^ Misler S (September 2009). "Unifying concepts in stimulus-secretion coupling in endocrine cells and some implications for therapeutics". Advances in Physiology Education. 33 (3): 175–86. doi:10.1152/advan.90213.2008. PMC 3747786. PMID 19745043.
- ^ Geracioti TD (2006). "Identifying Hypothyroidism's Psychiatric Presentations". Current Psychiatry. 5 (11): 98–117.
- ^ Huang JT, Leweke FM, Oxley D, Wang L, Harris N, Koethe D, et al. (November 2006). "Disease biomarkers in cerebrospinal fluid of patients with first-onset psychosis". PLOS Medicine. 3 (11): e428. doi:10.1371/journal.pmed.0030428. PMC 1630717. PMID 17090210.
- ^ a b c Leng, G.; Moos, F. C.; Armstrong, W. E. (2010-05-01). "The Adaptive Brain: Glenn Hatton and the Supraoptic Nucleus". Journal of Neuroendocrinology. 22 (5): 318–329. doi:10.1111/j.1365-2826.2010.01997.x. PMC 5713484. PMID 20298459.
- ^ Kandel, E. R. (1964-03-01). "Electrical Properties of Hypothalamic Neuroendocrine Cells". Journal of General Physiology. 47 (4): 691–717. doi:10.1085/jgp.47.4.691. ISSN 1540-7748. PMC 2195356. PMID 14127607.
- CS1 French-language sources (fr)
- Articles with short description
- Short description is different from Wikidata
- All articles with unsourced statements
- Articles with unsourced statements from May 2022
- Articles with unsourced statements from December 2013
- Articles with FAST identifiers
- Articles with BNF identifiers
- Articles with BNFdata identifiers
- Articles with GND identifiers
- Articles with J9U identifiers
- Articles with LCCN identifiers
- Articles with NKC identifiers
- Neuroendocrinology