Mitochondrial disease
| Mitochondrial disease | |
|---|---|
| Other names: Mitochondrial disorder,[1] mitochondrial cytopathy; mitochondriopathy (MCP) | |
 | |
| Summary of MD in clinical and pharmacological contexts, individuals with MD are characterized by involvement of different organs and tissues which results in various symptoms[2] | |
| Specialty | Medical genetics |
| Symptoms | Impaired vision, hearing loss, abnormal heartbeat, stunted growth[3] |
| Types | Are the following:[3] Barth syndrome Chronic progressive external ophthalmoplegia Kearns-Sayre syndrome Mitochondrial DNA depletion syndromes Mitochondrial neurogastrointestinal encephalomyopathy |
| Causes | Caused by mutations (acquired or inherited)[4][3] |
| Diagnostic method | EKG, medical history, CT, MRI[3] |
| Treatment | No cure or specific treatment ( focus is on managing symptoms)[3] |
Mitochondrial disease is a group of disorders caused by mitochondrial dysfunction. Mitochondria are the organelles that generate energy for the cell and are found in every cell of the human body except red blood cells. They convert the energy of food molecules into the ATP that powers most cell functions.[3][5][6]
Mitochondrial diseases take on unique characteristics both because of the way the diseases are often inherited and because mitochondria are so critical to cell function. A subclass of these diseases that have neuromuscular symptoms are known as mitochondrial myopathies.[3][7]
Types
Mitochondrial disease can manifest in many different ways[8] whether in children[9] or adults.[10] Examples of mitochondrial diseases include:
- Mitochondrial myopathy[9][10]
- Maternally inherited diabetes mellitus and deafness (MIDD)[11]
- While diabetes mellitus and deafness can be found together for other reasons, at an early age this combination can be due to mitochondrial disease, as may occur in Kearns–Sayre syndrome and Pearson syndrome[9]
- Leber's hereditary optic neuropathy (LHON)[10]
- Leigh syndrome, subacute necrotizing encephalomyelopathy[13]
- After normal development the disease usually begins late in the first year of life, although onset may occur in adulthood
- Rapid decline in function occurs and is marked by seizures, altered states of consciousness, dementia, ventilatory failure
- Neuropathy, ataxia, retinitis pigmentosa, and ptosis (NARP)[14]
- Progressive symptoms as described in the acronym
- Dementia
- Myoneurogenic gastrointestinal encephalopathy (MNGIE)[15]
- Gastrointestinal pseudo-obstruction
- Neuropathy
- MERRF syndrome[16]
- Progressive myoclonic epilepsy
- "Ragged Red Fibers" are clumps of diseased mitochondria that accumulate in the subsarcolemmal region of the muscle fiber and appear when muscle is stained with modified Gömöri trichrome stain
- Short stature
- Hearing loss
- Lactic acidosis
- Exercise intolerance
- MELAS syndrome, mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes[17]
- Mitochondrial DNA depletion syndrome[18]
Signs and symptoms
In terms of the presentation of Mitochondrial disorders we find that these vary depending on the type. However, there are some frequent symptoms and signs which are the following:[3]
- Impaired vision
- Hearing loss
- Abnormal heartbeat
- Stunted growth
- Neurological (and/or muscular issues )
Associated conditions
Acquired conditions in which mitochondrial dysfunction has been involved are:
- Diabetes[19]
- Huntington's disease[20]
- Cancer[21]
- Alzheimer's disease,[22]
- Parkinson's disease[23]
- Bipolar disorder,[24][25][26] schizophrenia, aging and senescence,[27] anxiety disorders[28]
- Cardiovascular disease[29]
- Sarcopenia[30]
- Chronic fatigue syndrome[25]
- ALS[31]
The body, and each mutation, is modulated by other genome variants; the mutation that in one individual may cause liver disease might in another person cause a brain disorder. The severity of the specific defect may also be great or small. Some defects include exercise intolerance. Defects often affect the operation of the mitochondria and multiple tissues more severely, leading to multi-system diseases.[32]
It has also been reported that drug tolerant cancer cells have an increased number and size of mitochondria, which suggested an increase in mitochondrial biogenesis.[33] Interestingly, a recent study in Nature Nanotechnology has reported that cancer cells can hijack the mitochondria from immune cells via physical tunneling nanotubes.[34]
As a rule, mitochondrial diseases are worse when the defective mitochondria are present in the muscles, cerebrum, or nerves, because these cells use more energy than most other cells in the body.Although mitochondrial diseases vary greatly in presentation from person to person, several major clinical categories of these conditions have been defined, based on the most common phenotypic features, symptoms, and signs associated with the particular mutations that tend to cause them.An outstanding question and area of research is whether ATP depletion or reactive oxygen species are in fact responsible for the observed phenotypic consequences.[35][additional citation(s) needed]
Cerebellar atrophy or hypoplasia has sometimes been reported to be associated.[36]
Causes

Mitochondrial disorders may be caused by mutations (acquired or inherited), in mitochondrial DNA (mtDNA), or in nuclear genes that code for mitochondrial components. They may also be the result of acquired mitochondrial dysfunction due to adverse effects of drugs, infections, or other environmental causes.[4]
Nuclear DNA has two copies per cell , one copy being inherited from the father and the other from the mother. Mitochondrial DNA, however, is inherited from the mother only and each mitochondrion typically contains between 2 and 10 mtDNA copies. During cell division the mitochondria segregate randomly between the two new cells. Those mitochondria make more copies, normally reaching 500 mitochondria per cell. As mtDNA is copied when mitochondria proliferate, they can accumulate random mutations, a phenomenon called heteroplasmy. If only a few of the mtDNA copies inherited from the mother are defective, mitochondrial division may cause most of the defective copies to end up in just one of the new mitochondria. Mitochondrial disease may become clinically apparent once the number of affected mitochondria reaches a certain level; this phenomenon is called "threshold expression".[37][38][additional citation(s) needed]
Mitochondria possess many of the same DNA repair pathways as nuclei do—but not all of them;[39] therefore, mutations occur more frequently in mitochondrial DNA than in nuclear DNA. This means that mitochondrial DNA disorders may occur spontaneously and relatively often. Defects in enzymes that control mitochondrial DNA replication may also cause mitochondrial DNA mutations.[40][41]
Most mitochondrial function and biogenesis is controlled by nuclear DNA. Human mitochondrial DNA encodes 13 proteins of the respiratory chain, while most of the estimated 1,500 proteins and components targeted to mitochondria are nuclear-encoded. Defects in nuclear-encoded mitochondrial genes are associated with hundreds of clinical disease phenotypes including anemia, dementia, hypertension, lymphoma, retinopathy, seizures, and neurodevelopmental disorders.[42]
A study by Yale University researchers (published in the February 12, 2004, issue of the New England Journal of Medicine) explored the role of mitochondria in insulin resistance among the offspring of patients with type 2 diabetes.[43] Other studies have shown that the mechanism may involve the interruption of the mitochondrial signaling process in body cells (intramyocellular lipids). A study conducted at the Pennington Biomedical Research Center in Baton Rouge, Louisiana[44] showed that this, in turn, partially disables the genes that produce mitochondria.
Mechanisms
The effective overall energy unit for the available body energy is referred to as the daily glycogen generation capacity,[45][46][47] and is used to compare the mitochondrial output of affected or chronically glycogen-depleted individuals to healthy individuals. This value is slow to change in a given individual, as it takes between 18 and 24 months to complete a full cycle.[46]
The glycogen generation capacity is entirely dependent on, and determined by, the operating levels of the mitochondria in all of the cells of the human body;[48] however, the relation between the energy generated by the mitochondria and the glycogen capacity is very loose and is mediated by many biochemical pathways.[45] The energy output of full healthy mitochondrial function can be predicted exactly by a complicated theoretical argument, however this argument is not straightforward[49]
Diagnosis

In terms of the diagnosis of Mitochondrial disorders we find the following:[3]
Mitochondrial diseases are usually detected by analysing muscle samples (where the presence of these organelles is higher).[50]
The most common tests for the detection of these diseases are:
- Southern blot to detect large deletions or duplications[51]
- Polymerase chain reaction and specific mutation testing[52]
- Sequencing[51]
Treatments
Although research is ongoing, treatment options are currently limited; vitamins are frequently prescribed, though the evidence for their effectiveness is limited.[53] Pyruvate has been proposed in 2007 as a treatment option.[54] N-acetyl cysteine reverses many models of mitochondrial dysfunction.[55] In the case of mood disorders, specifically bipolar disorder, it is hypothesized that N-acetyl-cysteine (NAC), acetyl-L-carnitine (ALCAR), S-adenosylmethionine (SAMe), coenzyme Q10 (CoQ10), alpha-lipoic acid (ALA), creatine monohydrate (CM), and melatonin could be potential treatment options.[56]
Mitochondrial replacement therapy
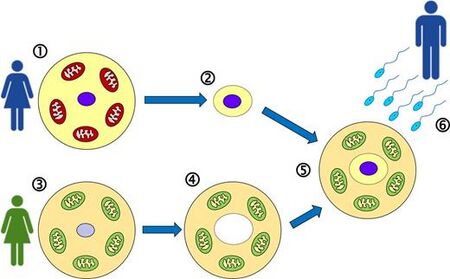
Jump to navigationJump to search Mitochondrial replacement therapy (MRT), where the nuclear DNA is transferred to another healthy egg cell leaving the defective mitochondrial DNA behind, is an IVF treatment procedure.[58] Using a similar pronuclear transfer technique, researchers at Newcastle University led by Douglass Turnbull successfully transplanted healthy DNA in human eggs from women with mitochondrial disease into the eggs of women donors who were unaffected.[59][60] In such cases, ethical questions have been raised regarding biological motherhood, since the child receives genes and gene regulatory molecules from two different women. Using genetic engineering in attempts to produce babies free of mitochondrial disease is controversial in some circles and raises important ethical issues.[61][62] A male baby was born in Mexico in 2016 from a mother with Leigh syndrome using MRT.[63]
In September 2012 a public consultation was launched in the UK to explore the ethical issues involved.[64] Human genetic engineering was used on a small scale to allow infertile women with genetic defects in their mitochondria to have children.[65] In June 2013, the United Kingdom government agreed to develop legislation that would legalize the 'three-person IVF' procedure as a treatment to fix or eliminate mitochondrial diseases that are passed on from mother to child. The procedure could be offered from 29 October 2015 once regulations had been established.[66][67][68] Embryonic mitochondrial transplant and protofection have been proposed as a possible treatment for inherited mitochondrial disease[69]
In 2018 Australian Senate's Senate Community Affairs References Committee recommended a move towards legalising Mitochondrial replacement therapy (MRT). Research and clinical applications of MRT were overseen by laws made by federal and state governments. State laws were, for the most part, consistent with federal law. In all states, legislation prohibited the use of MRT techniques in the clinic, and except for Western Australia, research on a limited range of MRT was permissible up to day 14 of embryo development, subject to a license being granted. In 2010, the Hon. Mark Butler MP, then Federal Minister for Mental Health and Ageing, had appointed an independent committee to review the two relevant acts: the Prohibition of Human Cloning for Reproduction Act 2002 and the Research Involving Human Embryos Act 2002. The committee's report, released in July 2011, recommended the existing legislation remain unchanged[70][additional citation(s) needed]
Epidemiology
About 1 in 4,000 children in the United States will develop mitochondrial disease by the age of 10 years. Up to 4,000 children per year in the US are born with a type of mitochondrial disease. Because mitochondrial disorders contain many variations and subsets, some particular mitochondrial disorders are very rare.[71][72]
The average number of births per year among women at risk for transmitting mtDNA disease is estimated to approximately 150 in the United Kingdom and 800 in the United States.[73]
History
The first pathogenic mutation in mitochondrial DNA was identified in 1988; from that time to 2016, around 275 other disease-causing mutations were identified.[74]
Society and culture

Notable people with mitochondrial disease include:
- Charles Darwin, a nineteenth century naturalist who suffered from a disabling illness, is speculated to have MELAS syndrome.[75]
- Mattie Stepanek, a poet, peace advocate, and motivational speaker who had dysautonomic mitochondrial myopathy, and who died at age 13.[76]
- Rocco Baldelli, a coach and former center fielder in Major League Baseball who had to retire from active play at age 29 due to mitochondrial channelopathy.[77][78][79]
- Charlie Gard, a British boy who had mitochondrial DNA depletion syndrome; decisions about his care were taken to various law courts.[80]
Research
Human clinical trials in 2014 at GenSight Biologics (ClinicalTrials.gov # NCT02064569) and the University of Miami (ClinicalTrials.gov # NCT02161380) to examine the safety and efficacy of mitochondrial gene therapy in Leber's hereditary optic neuropathy.[81]
References
- ↑ "Mitochondrial disease (Concept Id: C0751651) - MedGen - NCBI". www.ncbi.nlm.nih.gov. Archived from the original on 2024-04-09. Retrieved 2024-04-08.
- ↑ Soldatov, Vladislav O.; Kubekina, Marina V.; Skorkina, Marina Yu.; Belykh, Andrei E.; Egorova, Tatiana V.; Korokin, Mikhail V.; Pokrovskiy, Mikhail V.; Deykin, Alexey V.; Angelova, Plamena R. (5 December 2022). "Current advances in gene therapy of mitochondrial diseases". Journal of Translational Medicine. 20 (1): 562. doi:10.1186/s12967-022-03685-0. ISSN 1479-5876. Archived from the original on 30 October 2023. Retrieved 9 April 2024.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 "Mitochondrial Disorders | National Institute of Neurological Disorders and Stroke". www.ninds.nih.gov. Archived from the original on 2024-04-04. Retrieved 2024-04-09.
- ↑ 4.0 4.1 "Mitochondrial diseases". MeSH. Archived from the original on 9 December 2019. Retrieved 2 August 2019.
- ↑ Kanungo, S; Morton, J; Neelakantan, M; Ching, K; Saeedian, J; Goldstein, A (December 2018). "Mitochondrial disorders". Annals of translational medicine. 6 (24): 475. doi:10.21037/atm.2018.12.13. PMID 30740406. Archived from the original on 2023-03-24. Retrieved 2024-04-10.
- ↑ Schapira, Anthony HV (July 2006). "Mitochondrial disease". The Lancet. 368 (9529): 70–82. doi:10.1016/S0140-6736(06)68970-8. Archived from the original on 20 April 2013. Retrieved 11 April 2024.
- ↑ Ahuja, AS (2018). "Understanding mitochondrial myopathies: a review". PeerJ. 6: e4790. doi:10.7717/peerj.4790. PMID 29844960. Archived from the original on 2024-01-08. Retrieved 2024-04-12.
- ↑ "Mitochondrial Diseases". medlineplus.gov. Archived from the original on 2024-04-10. Retrieved 2023-03-15.
- ↑ 9.0 9.1 9.2 9.3 Rahman S (2020). "Mitochondrial disease in children". Journal of Internal Medicine. 287 (6): 609–633. doi:10.1111/joim.13054. PMID 32176382.
- ↑ 10.0 10.1 10.2 La Morgia C, Maresca A, Caporali L, Valentino ML, Carelli V (2020). "Mitochondrial diseases in adults". Journal of Internal Medicine. 287 (6): 592–608. doi:10.1111/joim.13064. PMID 32463135.
- ↑ Tsang SH, Aycinena AR, Sharma T (2018). "Mitochondrial disorder: maternally inherited diabetes and deafness". Atlas of Inherited Retinal Diseases. Advances in Experimental Medicine and Biology. Vol. 1085. pp. 163–165. doi:10.1007/978-3-319-95046-4_31. ISBN 978-3-319-95045-7. PMID 30578504.
- ↑ Shamsnajafabadi H, MacLaren RE, Cehajic-Kapetanovic J (2023). "Current and future landscape in genetic therapies for Leber hereditary optic neuropathy". Cells. 12 (15): 2013. doi:10.3390/cells12152013. PMID 37566092.
- ↑ Rahman S (2023). "Leigh syndrome". Mitochondrial Diseases. Handbook of Clinical Neurology. Vol. 194. pp. 43–63. doi:10.1016/B978-0-12-821751-1.00015-4. ISBN 9780128217511. PMID 36813320.
- ↑ "Neuropathy, ataxia, and retinitis pigmentosa: MedlinePlus Genetics". medlineplus.gov. Archived from the original on 2024-04-10. Retrieved 2024-04-10.
- ↑ Hirano, Michio (1993). "Mitochondrial Neurogastrointestinal Encephalopathy Disease". GeneReviews®. University of Washington, Seattle. Archived from the original on 2023-09-22. Retrieved 2024-04-11.
- ↑ Velez-Bartolomei, Frances; Lee, Chung; Enns, Gregory (1993). "MERRF". GeneReviews®. University of Washington, Seattle. Archived from the original on 2021-09-09. Retrieved 2024-04-11.
- ↑ "Orphanet: MELAS". www.orpha.net. Archived from the original on 15 April 2024. Retrieved 14 April 2024.
- ↑ Elpeleg, Orly (August 2003). "Inherited Mitochondrial DNA Depletion". Pediatric Research. 54 (2): 153–159. doi:10.1203/01.PDR.0000072796.25097.A5. ISSN 1530-0447. Archived from the original on 2 December 2023. Retrieved 11 April 2024.
- ↑ Maassen, J. Antonie; ‘t Hart, Leen. M.; van Essen, Einar; Heine, Rob J.; Nijpels, Giel; Jahangir Tafrechi, Roshan S.; Raap, Anton K.; Janssen, George M.C.; Lemkes, Herman H.P.J. (1 February 2004). "Mitochondrial Diabetes". Diabetes. 53 (suppl_1): S103–S109. doi:10.2337/diabetes.53.2007.S103. Archived from the original on 28 November 2022. Retrieved 13 April 2024.
- ↑ Kim, J.; Moody, J. P.; Edgerly, C. K.; Bordiuk, O. L.; Cormier, K.; Smith, K.; Beal, M. F.; Ferrante, R. J. (15 October 2010). "Mitochondrial loss, dysfunction and altered dynamics in Huntington's disease". Human Molecular Genetics. 19 (20): 3919–3935. doi:10.1093/hmg/ddq306. Archived from the original on 11 October 2022. Retrieved 13 April 2024.
- ↑ Poljsak, B.; Kovac, V.; Dahmane, R.; Levec, T.; Starc, A. (8 October 2019). "Cancer Etiology: A Metabolic Disease Originating from Life's Major Evolutionary Transition?". Oxidative Medicine and Cellular Longevity. 2019: e7831952. doi:10.1155/2019/7831952. ISSN 1942-0900. Archived from the original on 13 April 2024. Retrieved 13 April 2024.
- ↑ Abyadeh, Morteza; Gupta, Vivek; Chitranshi, Nitin; Gupta, Veer; Wu, Yunqi; Saks, Danit; WanderWall, Roshana; Fitzhenry, Matthew J; Basavarajappa, Devaraj; You, Yuyi; H Hosseini, Ghasem; A Haynes, Paul; L Graham, Stuart; Mirzaei, Mehdi (2021). "Mitochondrial dysfunction in Alzheimer's disease - a proteomics perspective". Expert Review of Proteomics. 18 (4): 295–304. doi:10.1080/14789450.2021.1918550. PMID 33874826. S2CID 233310698.
- ↑ Henrich, Martin T.; Oertel, Wolfgang H.; Surmeier, D. James; Geibl, Fanni F. (11 November 2023). "Mitochondrial dysfunction in Parkinson's disease – a key disease hallmark with therapeutic potential". Molecular Neurodegeneration. 18 (1): 83. doi:10.1186/s13024-023-00676-7. ISSN 1750-1326. Archived from the original on 16 February 2024. Retrieved 13 April 2024.
- ↑ Stork, C; Renshaw, P F (2005). "Mitochondrial dysfunction in bipolar disorder: Evidence from magnetic resonance spectroscopy research". Molecular Psychiatry. 10 (10): 900–19. doi:10.1038/sj.mp.4001711. PMID 16027739.
- ↑ 25.0 25.1 Pieczenik, Steve R; Neustadt, John (2007). "Mitochondrial dysfunction and molecular pathways of disease". Experimental and Molecular Pathology. 83 (1): 84–92. doi:10.1016/j.yexmp.2006.09.008. PMID 17239370.
- ↑ Nierenberg, Andrew A; Kansky, Christine; Brennan, Brian P; Shelton, Richard C; Perlis, Roy; Iosifescu, Dan V (2012). "Mitochondrial modulators for bipolar disorder: A pathophysiologically informed paradigm for new drug development". Australian & New Zealand Journal of Psychiatry. 47 (1): 26–42. doi:10.1177/0004867412449303. PMID 22711881. S2CID 22983555.
- ↑ Valiente-Pallejà, A; Tortajada, J; Bulduk, BK (2022). "Comprehensive summary of mitochondrial DNA alterations in the postmortem human brain: A systematic review". eBioMedicine. 76 (103815): 103815. doi:10.1016/j.ebiom.2022.103815. PMC 8790490. PMID 35085849.
- ↑ Misiewicz, Zuzanna; Iurato, Stella; Kulesskaya, Natalia; Salminen, Laura; Rodrigues, Luis; Maccarrone, Giuseppina; Martins, Jade; Czamara, Darina; Laine, Mikaela A.; Sokolowska, Ewa; Trontti, Kalevi; Rewerts, Christiane; Novak, Bozidar; Volk, Naama; Park, Dong Ik; Jokitalo, Eija; Paulin, Lars; Auvinen, Petri; Voikar, Vootele; Chen, Alon; Erhardt, Angelika; Turck, Christoph W.; Hovatta, Iiris (26 September 2019). "Multi-omics analysis identifies mitochondrial pathways associated with anxiety-related behavior". PLOS Genetics. 15 (9): e1008358. doi:10.1371/journal.pgen.1008358. PMC 6762065. PMID 31557158.
- ↑ Yang, Jiaqi; Guo, Qianyun; Feng, Xunxun; Liu, Yang; Zhou, Yujie (13 May 2022). "Mitochondrial Dysfunction in Cardiovascular Diseases: Potential Targets for Treatment". Frontiers in Cell and Developmental Biology. 10. doi:10.3389/fcell.2022.841523. Archived from the original on 15 April 2024. Retrieved 13 April 2024.
- ↑ Kim, Min-Ji; Sinam, Ibotombi Singh; Siddique, Zerwa; Jeon, Jae-Han; Lee, In-Kyu (31 March 2023). "The Link between Mitochondrial Dysfunction and Sarcopenia: An Update Focusing on the Role of Pyruvate Dehydrogenase Kinase 4" (PDF). Diabetes & Metabolism Journal. 47 (2): 153–163. doi:10.4093/dmj.2022.0305. Archived (PDF) from the original on 15 April 2024. Retrieved 13 April 2024.
- ↑ Muyderman, H; Chen, T (April 2014). "Mitochondrial dysfunction in amyotrophic lateral sclerosis – a valid pharmacological target?". British Journal of Pharmacology. 171 (8): 2191–2205. doi:10.1111/bph.12476. PMC 3976630. PMID 24148000.
- ↑ Nunnari J, Suomalainen A (2012). "Mitochondria: in sickness and in health". Cell. 148 (6): 1145–59. doi:10.1016/j.cell.2012.02.035. PMC 5381524. PMID 22424226.
- ↑ Goldman A, Khiste S, Freinkman E, Dhawan A, Majumder B, Mondal J, et al. (August 2019). "Targeting tumor phenotypic plasticity and metabolic remodeling in adaptive cross-drug tolerance". Science Signaling. 12 (595). doi:10.1126/scisignal.aas8779. PMC 7261372. PMID 31431543.
- ↑ Saha T, Dash C, Jayabalan R, et al. (2021). "Intercellular nanotubes mediate mitochondrial trafficking between cancer and immune cells". Nat. Nanotechnol. 17 (1): 98–106. doi:10.1038/s41565-021-01000-4. PMID 34795441. S2CID 244349825.
- ↑ Finsterer, Josef (2007). "Hematological Manifestations of Primary Mitochondrial Disorders". Acta Haematologica. 118 (2): 88–98. doi:10.1159/000105676. PMID 17637511. S2CID 24222021.
- ↑ Lax, Nichola Zoe; Hepplewhite, Philippa Denis; Reeve, Amy Katherine; Nesbitt, Victoria; McFarland, Robert; Jaros, Evelyn; Taylor, Robert William; Turnbull, Douglass Matthew (2012). "Cerebellar Ataxia in Patients with Mitochondrial DNA Disease". Journal of Neuropathology & Experimental Neurology. 71 (2): 148–61. doi:10.1097/NEN.0b013e318244477d. PMC 3272439. PMID 22249460.
- ↑ Wei, W; Chinnery, PF (June 2020). "Inheritance of mitochondrial DNA in humans: implications for rare and common diseases". Journal of internal medicine. 287 (6): 634–644. doi:10.1111/joim.13047. PMID 32187761. Archived from the original on 2024-04-10. Retrieved 2024-04-15.
- ↑ Craven L, Alston CL, Taylor RW, Turnbull DM (August 2017). "Recent Advances in Mitochondrial Disease". Annual Review of Genomics and Human Genetics. 18 (1): 257–275. doi:10.1146/annurev-genom-091416-035426. PMID 28415858.
- ↑ Alexeyev M, Shokolenko I, Wilson G, LeDoux S (May 2013). "The maintenance of mitochondrial DNA integrity--critical analysis and update". Cold Spring Harbor Perspectives in Biology. 5 (5): a012641. doi:10.1101/cshperspect.a012641. PMC 3632056. PMID 23637283.
- ↑ Orsucci, D; Caldarazzo Ienco, E; Rossi, A; Siciliano, G; Mancuso, M (17 March 2021). "Mitochondrial Syndromes Revisited". Journal of clinical medicine. 10 (6). doi:10.3390/jcm10061249. PMID 33802970. Archived from the original on 18 April 2024. Retrieved 16 April 2024.
- ↑ Copeland, WC (2008). "Inherited mitochondrial diseases of DNA replication". Annual review of medicine. 59: 131–46. doi:10.1146/annurev.med.59.053006.104646. PMID 17892433. Archived from the original on 2024-04-13. Retrieved 2024-04-16.
- ↑ Scharfe C, Lu HH, Neuenburg JK, Allen EA, Li GC, Klopstock T, Cowan TM, Enns GM, Davis RW (2009). Rzhetsky A (ed.). "Mapping gene associations in human mitochondria using clinical disease phenotypes". PLOS Comput Biol. 5 (4): e1000374. Bibcode:2009PLSCB...5E0374S. doi:10.1371/journal.pcbi.1000374. PMC 2668170. PMID 19390613.
- ↑ Petersen, Kitt Falk; Dufour, Sylvie; Befroy, Douglas; Garcia, Rina; Shulman, Gerald I. (12 February 2004). "Impaired Mitochondrial Activity in the Insulin-Resistant Offspring of Patients with Type 2 Diabetes". New England Journal of Medicine. 350 (7): 664–671. doi:10.1056/NEJMoa031314. PMC 2995502. PMID 14960743.
- ↑ Sparks, Lauren M.; Xie, Hui; Koza, Robert A.; Mynatt, Randall; Hulver, Matthew W.; Bray, George A.; Smith, Steven R. (July 2005). "A High-Fat Diet Coordinately Downregulates Genes Required for Mitochondrial Oxidative Phosphorylation in Skeletal Muscle". Diabetes. 54 (7): 1926–1933. doi:10.2337/diabetes.54.7.1926. PMID 15983191. Gale A134380159 ProQuest 216493144.
- ↑ 45.0 45.1 Mitchell, Peter. "David Keilin's respiratory chain concept and its chemiosmotic consequences" (PDF). Nobel institute. Archived (PDF) from the original on 2018-06-12. Retrieved 2024-03-20.
- ↑ 46.0 46.1 Michelakis, Evangelos (January 2007). "A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth". University of Alberta. University of Alberta, 2007. 11 (1): 37–51. doi:10.1016/j.ccr.2006.10.020. PMID 17222789.
- ↑ Lorini & Ciman, M, & M (1962). "Hypoglycaemic action of Diisopropylammonium salts in experimental diabetes". Institute of Biochemistry, University of Padua, September 1962. Biochemical Pharmacology. 11 (9): 823–827. doi:10.1016/0006-2952(62)90177-6. PMID 14466716.
- ↑ Stacpoole PW, Henderson GN, Yan Z, James MO (1998). "Clinical pharmacology and toxicology of dichloroacetate". Environ. Health Perspect. 106 (Suppl 4): 989–94. doi:10.1289/ehp.98106s4989. PMC 1533324. PMID 9703483.
- ↑ Hoitzing, Hanne; Johnston, Iain G.; Jones, Nick S. (June 2015). "What is the function of mitochondrial networks? A theoretical assessment of hypotheses and proposal for future research". BioEssays. 37 (6): 687–700. doi:10.1002/bies.201400188.
- ↑ Muraresku, CC; McCormick, EM; Falk, MJ (June 2018). "Mitochondrial Disease: Advances in clinical diagnosis, management, therapeutic development, and preventative strategies". Current genetic medicine reports. 6 (2): 62–72. doi:10.1007/s40142-018-0138-9. PMID 30393588. Archived from the original on 2023-05-24. Retrieved 2024-04-17.
- ↑ 51.0 51.1 Parikh, Sumit; Goldstein, Amy; Koenig, Mary Kay; Scaglia, Fernando; Enns, Gregory M.; Saneto, Russell; Anselm, Irina; Cohen, Bruce H.; Falk, Marni J.; Greene, Carol; Gropman, Andrea L.; Haas, Richard; Hirano, Michio; Morgan, Phil; Sims, Katherine; Tarnopolsky, Mark; Van Hove, Johan L.K.; Wolfe, Lynne; DiMauro, Salvatore (September 2015). "Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society". Genetics in Medicine. 17 (9): 689–701. doi:10.1038/gim.2014.177. Archived from the original on 2023-05-17. Retrieved 2024-04-17.
- ↑ Bulduk, Bengisu Kevser; Kiliç, Hasan Basri; Bekircan-Kurt, Can Ebru; Haliloğlu, Göknur; Erdem Özdamar, Sevim; Topaloğlu, Haluk; Kocaefe, Y. Çetin (March 2020). "A Novel Amplification-Refractory Mutation System-PCR Strategy to Screen MT-TL1 Pathogenic Variants in Patient Repositories". Genetic Testing and Molecular Biomarkers. 24 (3): 165–170. doi:10.1089/gtmb.2019.0079. PMID 32167396. S2CID 212693790.
- ↑ Marriage B, Clandinin MT, Glerum DM (2003). "Nutritional cofactor treatment in mitochondrial disorders". J Am Diet Assoc. 103 (8): 1029–38. doi:10.1016/S0002-8223(03)00476-0. PMID 12891154.
- ↑ Tanaka M, Nishigaki Y, Fuku N, Ibi T, Sahashi K, Koga Y (2007). "Therapeutic potential of pyruvate therapy for mitochondrial diseases". Mitochondrion. 7 (6): 399–401. doi:10.1016/j.mito.2007.07.002. PMID 17881297.
- ↑ Frantz MC, Wipf P (Jun 2010). "Mitochondria as a target in treatment". Environ Mol Mutagen. 51 (5): 462–75. Bibcode:2010EnvMM..51..462F. doi:10.1002/em.20554. PMC 2920596. PMID 20175113.
- ↑ Nierenberg, Andrew A, Kansky, Christine, Brennan, Brian P, Shelton, Richard C, Perlis, Roy, Iosifescu, Dan V (2012). "Mitochondrial modulators for bipolar disorder: A pathophysiologically informed paradigm for new drug development". Australian & New Zealand Journal of Psychiatry. 47 (1): 26–42. doi:10.1177/0004867412449303. PMID 22711881. S2CID 22983555.
- ↑ Jackson, Maria; Marks, Leah; May, Gerhard H.W.; Wilson, Joanna B. (3 December 2018). "The genetic basis of disease". Essays in Biochemistry. 62 (5): 643–723. doi:10.1042/EBC20170053.
- ↑ Tachibana M, Sparman M, Sritanaudomchai H, Ma H, Clepper L, Woodward J, Li Y, Ramsey C, Kolotushkina O, Mitalipov S (September 2009). "Mitochondrial gene replacement in primate offspring and embryonic stem cells". Nature. 461 (7262): 367–372. Bibcode:2009Natur.461..367T. doi:10.1038/nature08368. PMC 2774772. PMID 19710649.
- ↑ Boseley, Sarah (2010-04-14). "Scientists reveal gene-swapping technique to thwart inherited diseases". Guardian. London. Archived from the original on 2023-11-21. Retrieved 2024-03-20.
- ↑ Craven, Lyndsey; Tuppen, Helen A.; Greggains, Gareth D.; Harbottle, Stephen J.; Murphy, Julie L.; Cree, Lynsey M.; Murdoch, Alison P.; Chinnery, Patrick F.; Taylor, Robert W.; Lightowlers, Robert N.; Herbert, Mary; Turnbull, Douglass M. (2010). "Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease". Nature. 465 (7294): 82–85. Bibcode:2010Natur.465...82C. doi:10.1038/nature08958. PMC 2875160. PMID 20393463.

- ↑ "UK urged to permit IVF procedure to prevent fatal genetic diseases". The Guardian. London. 2015-04-30. Archived from the original on 2023-12-10. Retrieved 2024-03-20.
- ↑ "Three parent baby law is 'irresponsible' says Church of England ahead of vote". The Telegraph. London. 2015-04-30. Archived from the original on 2024-01-14. Retrieved 2024-03-20.
- ↑ Hamzelou, Jessica (2016-09-27). "Exclusive: World's first baby born with new "3 parent" technique". New Scientist. Archived from the original on 2019-05-03. Retrieved 2016-11-26.
- ↑ Sample, Ian (2012-09-17). "Regulator to consult public over plans for new fertility treatments". The Guardian. London. Archived from the original on 2014-05-02. Retrieved 8 October 2012.
- ↑ "Genetically altered babies born". BBC News. 2001-05-04. Archived from the original on 2011-10-10. Retrieved 2008-04-26.
- ↑ "The Human Fertilisation and Embryology (Mitochondrial Donation) Regulations 2015". Archived from the original on 2023-12-20. Retrieved 2024-03-20.
- ↑ "UK government backs three-person IVF". BBC News. 27 June 2013. Archived from the original on 30 June 2013. Retrieved 20 March 2024.
- ↑ Knapton, Sarah (1 March 2014) 'Three-parent babies' could be born in Britain next year The Daily Telegraph Science News, Retrieved 1 March 2014
- ↑ Clemente-Suárez, Vicente Javier; Martín-Rodríguez, Alexandra; Yáñez-Sepúlveda, Rodrigo; Tornero-Aguilera, José Francisco (January 2023). "Mitochondrial Transfer as a Novel Therapeutic Approach in Disease Diagnosis and Treatment". International Journal of Molecular Sciences. 24 (10): 8848. doi:10.3390/ijms24108848. ISSN 1422-0067.
- ↑ "Science of mitochondrial donation and related matters". www.aph.gov.au. Archived from the original on 10 December 2023. Retrieved 14 April 2024.
- ↑ Amato, Paula; Tachibana, Masahito; Sparman, Michelle; Mitalipov, Shoukhrat (January 2014). "Three-parent in vitro fertilization: gene replacement for the prevention of inherited mitochondrial diseases". Fertility and Sterility. 101 (1): 31–35. doi:10.1016/j.fertnstert.2013.11.030. PMID 24382342.
- ↑ Buajitti, Emmalin; Rosella, Laura C.; Zabzuni, Ersi; Young, L. Trevor; Andreazza, Ana C. (8 April 2022). "Prevalence and health care costs of mitochondrial disease in Ontario, Canada: A population-based cohort study". PLOS ONE. 17 (4): e0265744. doi:10.1371/journal.pone.0265744.
- ↑ Gorman, Gráinne S.; Grady, John P.; Ng, Yi; Schaefer, Andrew M.; McNally, Richard J.; Chinnery, Patrick F.; Yu-Wai-Man, Patrick; Herbert, Mary; Taylor, Robert W.; McFarland, Robert; Turnbull, Doug M. (26 February 2015). "Mitochondrial Donation — How Many Women Could Benefit?". New England Journal of Medicine. 372 (9): 885–887. doi:10.1056/NEJMc1500960. PMC 4481295. PMID 25629662.
- ↑ Claiborne, A.; English, R.; Kahn, J. (2016). "Etiology, Clinical Manifestation, and Diagnosis". In Claiborne, Anne; English, Rebecca; Kahn, Jeffrey (eds.). Mitochondrial Replacement Techniques. p. 37. doi:10.17226/21871. ISBN 978-0-309-38870-2. PMID 27054230.
- ↑ Hayman, John (May 2013). "Charles Darwin's Mitochondria". Genetics. 194 (1): 21–25. doi:10.1534/genetics.113.151241. PMC 3632469. PMID 23633139.
- ↑ "Young poet, peace advocate Mattie dies | The Spokesman-Review". www.spokesman.com. Archived from the original on 2023-09-27. Retrieved 2024-03-20.
- ↑ Casey, Tim (May 18, 2015). "The Incredible Comeback of Rocco Baldelli, Baseball's Lost Star | VICE Sports". VICE Sports. Archived from the original on May 18, 2015. Retrieved March 28, 2021.
- ↑ McDonald, Joe (November 16, 2016). "Tampa Bay Rays coach Rocco Baldelli knows what Carolina Hurricanes' Bryan Bickell is up against". ABC News. Archived from the original on June 11, 2022. Retrieved June 11, 2022.
- ↑ Topkin, Mark (August 25, 2005). "Baldelli beat rare but treatable Lyme disease". Tampa Bay Times. Archived from the original on June 11, 2022. Retrieved June 11, 2022.
- ↑ Paris, J. J.; Ahluwalia, J.; Cummings, B. M.; Moreland, M. P.; Wilkinson, D. J. (December 2017). "The Charlie Gard case: British and American approaches to court resolution of disputes over medical decisions". Journal of Perinatology. pp. 1268–1271. doi:10.1038/jp.2017.138. Archived from the original on 2022-10-06. Retrieved 2024-04-16.
- ↑ "Safety Evaluation of Gene Therapy in Leber Hereditary Optic Neuropathy (LHON) Patients - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Archived from the original on 18 April 2024. Retrieved 16 April 2024.
External links
- Mitochondrial disease at Curlie
- International Mito Patients (IMP) Archived 2023-12-07 at the Wayback Machine
| Classification | |
|---|---|
| External resources |
- Pages with script errors
- All articles needing additional references
- Articles needing additional references from October 2017
- Articles with invalid date parameter in template
- Articles needing additional references
- Articles with Curlie links
- Webarchive template wayback links
- Mitochondrial diseases
- Molecular biology
- Mitochondrial genetics