Homoaconitic acid
Jump to navigation
Jump to search
 cis-Homoaconitic acid
| |
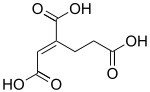 trans-Homoaconitic acid
| |
| Names | |
|---|---|
| IUPAC names
(1Z)-1-Butene-1,2,4-tricarboxylic acid
(1E)-1-Butene-1,2,4-tricarboxylic acid | |
| Other names
Homo-cis-aconitate; Homo-trans-aconitate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider |
|
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C7H8O6 | |
| Molar mass | 188.135 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Homoaconitatic acid (homoaconitate) is related to aconitic acid but with one extra carbon. It is part of the α-aminoadipate pathway for lysine biosynthesis, where it is made from homocitrate by homoaconitase.[1] It is converted to homoisocitrate by homoisocitrate dehydrogenase.
See also
References
- ^ Murray Strassman and Louis N. Ceci (1966). "Enzymatic Formation of cis-Homoaconitic Acid, an Intermediate in Lysine Biosynthesis in Yeast". The Journal of Biological Chemistry. 241 (22): 5401–5407. doi:10.1016/S0021-9258(18)96444-6. PMID 5954805.
Categories:
- Chemical articles with multiple compound IDs
- Multiple chemicals in an infobox that need indexing
- Chemical articles with multiple CAS registry numbers
- Articles without InChI source
- Articles without EBI source
- Articles without KEGG source
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Short description matches Wikidata
- Tricarboxylic acids
- All stub articles
- Organic compound stubs