Group A streptococcal infection
Group A streptococcal infection, also known as strep A, is a bacterial infection with group A streptococcus (GAS, Streptococcus pyogenes).[1] Individuals may have GAS on their skin or throat and show no symptoms.[1] In others it can lead to sepsis.[1] It may present as pharyngitis, impetigo, scarlet fever, erysipelas, or cellulitis.[1] Features of more severe disease such as pneumonia, bacteremia, necrotising fasciitis, puerperal sepsis, toxic shock syndrome, or myonecrosis may occur.[1] Complications include rheumatic fever, kidney disease, and reactive arthritis.[1]
Infection typically spreads through direct contact with respiratory droplets or sores on the skin; spread via food and water is less frequent.[6] Diagnosis may include cultures from the throat, tissue, blood, or sputum.[3] Blood tests may include complete blood count, ASO titre, ESR, and CRP.[3] Other tests, depending on symptoms, might include chest X-ray.[1]
Treatment is with antibiotics.[2] Penicillin and amoxicillin by mouth are recommended to reduce spread and complications of Strep throat, and to treat erysipelas and extensive impetigo.[1][4] Where there is penicillin allergy or a shortage of penicillin, alternatives include cefalexin, cefadroxil, clindamycin, azithromycin and clarithromycin.[4][5] Applying 2% mupirocin to small areas of impetigo and ecthyma can reduce spread to others.[1] For more severe GAS infections, antibiotics, typically penicillin, is administered via injection into veins.[1] Where it is difficult to distinguish between Staph and Strep infections, options include cefalexin, clindamycin, trimethoprim/sulfamethoxazole or vancomycin with ceftazidime.[1]
GAS infections occur most frequently in children younger than 10 years; up to a fifth of children are carriers without symptoms.[1] It results in over half a million deaths per year.[7] It is an increasing problem, particularly in Africa.[8] It is more common in during winter and where there is overcrowding.[1] These diseases have been recognized for over 2,000 years.[9]
Signs and symptoms
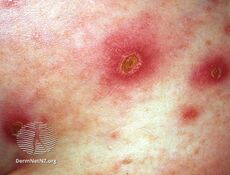
GAS typically cause infections of the throat and skin; a Strep throat, impetigo, cellulitis, and erysipelas.[1][10] These may vary from mild conditions to severe, life-threatening diseases.[11] Individuals may also carry the GAS either on the skin or in the throat and show no symptoms.[12] These carriers are less contagious than symptomatic carriers of the bacteria.[12] The non-invasive infections caused by GAS tend to be less severe and more common.[1] The invasive infections caused by GAS tend to be more severe and less common. These occurs when the bacterium is able to infect areas where bacteria are not usually found, such as blood and organs.[12] The diseases that may be caused as a result of this include streptococcal toxic shock syndrome (STSS), necrotizing fasciitis (NF), pneumonia, and bacteremia.[7] Severe Group A streptococcal infections often occur sporadically but can be spread by person-to-person contact.[13]
Less frequently it may occur as bacteremia,[14] septic arthritis,[15] osteomyelitis,[16] meningitis,[17] or sinusitis.[18] It may cause vaginitis.[19] or pneumonia.[20]
Strep throat
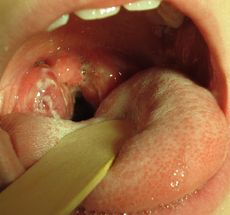
GAS infections most frequently present as a Strep throat in a child of school age, with a sudden onset of fever, sore throat and swollen neck glands; typically without cough and runny nose.[1] The uvula may look swollen and red and the tonsils typically appear swollen with exudate.[1] There may be small red spots on the soft palate.[1]
-
Strep throat with spots on soft palate, red uvula and strawberry tongue
-
Strep throat with spots on soft palate, red uvula and red pharynx
-
Strep throat with large tonsils covered in exudate
Scarlet fever
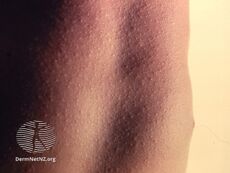
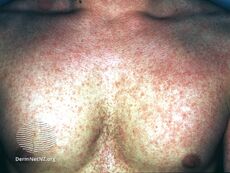
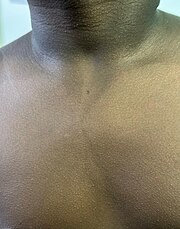
Scarlet fever typically occurs following a sore throat and presents with a sandpapery rash.[21]
-
The rash in scarlet fever
-
Scarlet fever rash
-
Scarlet fever rash on trunk
-
Rash of scarlet fever in dark skin
-
Facial flushing and classic rash of scarlet fever in light skin
Impetigo
Impetigo caused by GAS typically occurs in children age 2 to 5 years in the summers of temperate climates and all year around in tropical climates.[1] Risk factors include poor hygiene and malnutrition.[1] GAS that colonises unbroken skin enters the skin through a cut or abrasion and within 2 weeks several golden crusted lesions appear. [1] An ulcerative type of impetigo is ecthyma.[1] A swab for culture may help differentiate GAS impetigo from staphylococcal impetigo.[1]
-
Ecthyma
Cellulitis
GAS cellulitis typically does not have pus, like staphylococcal cellulitis.[1] The skin is red with a less defined border than that of erysipelas.[1] Venous insufficiency, poor lymph drainage, obesity, edema, tinea pedis and wounds are risk factors.[1]
Erysipelas
When erysipelas is caused by GAS, it generally presents with a rapid onset of painful well defined red skin typically of the face or hands and feet.[1] It may look raised and characteristically does not have golden crusts.[1]
Severe disease
Pneumonia
GAS pneumonia typically occurs in a person who already has a chronic lung problem or has recently had a strep. upper respiratory tract infection.[1] Symptoms of fever, cough, difficulty breathing, chills, chest pain and blood stained sputum appear suddenly.[1] Around a third of cases may develop empyema.[1]
Bacteremia
Severe infections are usually invasive, meaning that the bacteria has entered parts of the body where bacteria are not usually found, such as the blood, lungs, deep muscle or fat tissue.[1]
Puerperal sepsis
Puerperal sepsis caused by GAS typically presents with abdominal pain, fever, low blood pressure.[1]
Necrotising fasciitis
Necrotising fasciitis caused by GAS typically starts at the site of a small wound and develops into infection deep in skin and underlying tissue.[1]
Toxic shock syndrome
Frequently preceded by necrotising fasciitis or pneumonia, GAS toxic shock syndrome (TSS) appears similar to staphylococcal TSS and may begin with mild symptoms of fever, nausea, chills, vomiting and diarrhoea, followed 12 to 36 hours later by a sudden onset of low blood pressure and kidney failure.[22] It generally occurs in all ages, and particularly in people with diabetes or alcoholism.[22] Unlike Staph TSS, there is typically severe pain in GAS TSS, with bacteremia, a history of skin trauma or chicken pox, and a higher mortality.[22] The generalised red rash that is frequently seen in Staph TSS is less common in GAS TSS.[22] If there is a rash, the skin typically peels around a week after onset of symptoms.[22]
Myonecrosis
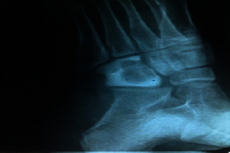
GAS infected muscle and surrounding structures.[1]
-
GAS bone
Complications
Complications include acute rheumatic fever and post-streptococcal glomerulonephritis.[1] Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) is another complication.[2][23]
All severe GAS infections may lead to shock, multisystem organ failure, and death.[24][25]
Acute rheumatic fever
Acute rheumatic fever (ARF) is a complication of respiratory infections caused by GAS.[26] The M-protein generates antibodies that cross-react with autoantigens on interstitial connective tissue, in particular of the endocardium and synovium, that can lead to significant clinical illness.Although common in developing countries, ARF is rare in the United States, possibly secondary to improved antibiotic treatment, with small isolated outbreaks reported only occasionally. It is most common among children between 5 and 15 years old and occurs after an untreated GAS pharyngitis.[2][27][28]
ARF is often clinically diagnosed based on Jones criteria, which include: pancarditis, migratory polyarthritis of large joints, subcutaneous nodules, erythema marginatum, and sydenham chorea (involuntary, purposeless movement). The most common clinical finding is a migratory arthritis involving multiple joints.[29]
Other indicators of GAS infection such as a DNAase or ASO serology test must confirm the GAS infection. Other minor Jones Criteria are fever, elevated ESR and arthralgia. One of the most serious complications is pancarditis, or inflammation of all three heart tissues. A fibrinous pericarditis can develop with a classic friction rub that can be auscultated.[30][28]
Further endocarditis can develop with aseptic vegetations along the valve closure lines, in particular the mitral valve. Chronic rheumatic heart disease mostly affects the mitral valve, which can become thickened with calcification of the leaflets, often causing fusion of the commissures and chordae tendineae.[31][32]
Other findings of ARF include erythema marginatum and a red expanding rash on the trunk and extremities that recurs over weeks to months. Because of the different ways ARF presents itself, the disease may be difficult to diagnose.[33]
A neurological disorder, Sydenham chorea, can occur months after an initial attack, causing jerky involuntary movements, muscle weakness, slurred speech, and personality changes. Initial episodes of ARF, as well as recurrences, can be prevented by treatment with appropriate antibiotics.[34][35]
It is important to distinguish ARF from rheumatic heart disease. ARF is an acute inflammatory reaction with pathognomonic Aschoff bodies histologically and RHD is a non-inflammatory sequela of ARF.[36]
Glomerulonephritis
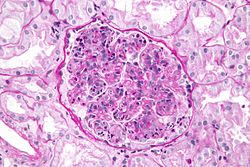
Post-streptococcal glomerulonephritis (PSGN) is an uncommon complication of either a strep throat or a streptococcal skin infection. It is classified as a type III hypersensitivity reaction. Symptoms of PSGN develop within 10 days following a strep throat or 3 weeks following a GAS skin infection. PSGN involves inflammation of the kidney. Symptoms include pale skin, lethargy, loss of appetite, headache, and dull back pain. Clinical findings may include dark-colored urine, swelling of different parts of the body (edema), and high blood pressure. Treatment of PSGN consists of supportive care.[37][38][needs copy edit]
PANDAS
Obsessive–compulsive disorder and tic disorders are hypothesized to arise in a subset of children as a result of a post-streptococcal autoimmune process.[39][40][41] Its potential effect was described in 1998 by the controversial hypothesis called PANDAS (pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections), a condition thought to be triggered by GABHS infections.[42][43] The PANDAS hypothesis is unconfirmed and unsupported by data, and two new categories have been proposed: PANS (pediatric acute-onset neuropsychiatric syndrome) and CANS (childhood acute neuropsychiatric syndrome).[40][41] The CANS/PANS hypotheses include different possible mechanisms underlying acute-onset neuropsychiatric conditions, but do not exclude GABHS infections as a cause in a subset of individuals.[40][41] PANDAS, PANS and CANS are the focus of clinical and laboratory research but remain unproven.[39][40][41]
Cause
'Streptococcus pyogenes comprises the vast majority of the Lancefield group A streptococci, and is often used as a synonym for GAS. However, S. dysgalactiae can also be group A.[44] S. pyogenes is a beta-hemolytic species of Gram positive bacteria that is responsible for a wide range of both invasive and noninvasive infections.[45] Several virulence factors contribute to the pathogenesis of GAS, such as M protein, hemolysins, and extracellular enzymes. Other types of streptococci include group B streptococcus (Streptococcus agalactiae) and Streptococcus pneumoniae, which cause other types of infections.[46][47]
The etiology of this infection finds that Streptococcus pyogenes is a species of Gram-positive, aerotolerant bacteria in the genus Streptococcus. These bacteria are extracellular, and made up of non-motile and non-sporing cocci that tend to link in chains. They are clinically important for humans, as they are an infrequent, but usually pathogenic, part of the skin microbiota that can cause Group A streptococcal infection [48]
Although it is not completely clear what causes different people to develop different diseases as a result of infection with the same pathogenic bacteria, it is suspected that host phenotypic and epigenetic factors are the source of such variation. Indeed, the many virulence factors of GAS can influence the epigenetics of the host. Furthermore, persons with suppressed or compromised immune systems may be more susceptible to certain diseases caused by GAS than other persons with intact immune systems. A 2019 study shows that GAS's evasion of immune detection is facilitated by protein S, an extracellular and cell wall-associated protein that enables it to camouflage itself by binding fragments of lysed red blood cells.[49]
Strep throat occurs when the bacteria colonizes the throat area, where it recognizes epithelial cells.[50]
-
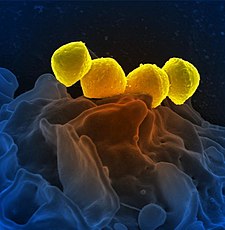
Scanning electron micrograph of Group A Streptococcus (Streptococcus pyogenes) bacteria yellow and a human neutrophil blue’’
-
Photomicrograph of Streptococcus pyogenes
-
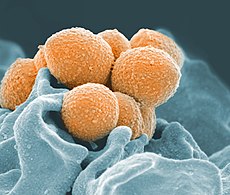
Scanning electron microscope image of Group A Streptococcus orange during phagocytic interaction with a human neutrophil teal
-
Scanning electron micrograph of Streptococcus pyogenese bacteria yellow bound to a human neutrophil blue
Risk factors
Close contacts of people affected by severe Group A streptococcal infections, defined as those having had prolonged household contact in the week before the onset of illness, may be at increased risk of infection. This increased risk may be due to a combination of shared genetic susceptibility within the family, close contact with carriers, and the virulence of the Group A streptococcal strain that is involved.[51]
Those at greatest risk include children with chickenpox; persons with suppressed immune systems; elderly persons with cellulitis, diabetes, vascular disease, or cancer; and persons taking steroid treatments or chemotherapy. Intravenous drug users also are at high risk. GAS is an important cause of puerperal fever worldwide, causing serious infection and, if not promptly diagnosed and treated, death in newly delivered mothers. Severe GAS disease may also occur in healthy persons with no known risk factors.[52][53][54]
Mechanism
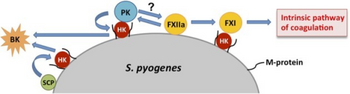
In terms of the mechanism of this infection one finds that a ability by group A streptococcus to fight off phagocytosis is apparently related to factor H and fibrinogen binding by M protein, as well as to disarming complement component C5a.[55]
Immunological response to streptococcal infection is via an abundance of antibodies in opposition to streptococcal cellular/extracellular components[55]
Diagnosis
Diagnosis is by a swab of the affected area for laboratory testing. A Gram stain is performed to show Gram-positive cocci in chains. Then, the organism is cultured on blood agar with an added bacitracin antibiotic disk to show beta-hemolytic colonies and sensitivity (zone of inhibition around the disk) for the antibiotic. Culture on agar not containing blood, and then performing the catalase test should show a negative reaction for all streptococci. S. pyogenes is CAMP and hippurate tests negative. Serological identification of the organism involves testing for the presence of group-A-specific polysaccharide in the bacterium's cell wall using the Phadebact test.[56][57]
The rapid pyrrolidonyl arylamidase (PYR) test is used for the presumptive identification of group A beta-hemolytic streptococci. GBS gives a negative finding on this test.[58]
Prevention
S. pyogenes infections are best prevented through effective hand hygiene.[59] No vaccines are currently available to protect against S. pyogenes infection, although research has been conducted into the development of one.[60] Difficulties in developing a vaccine include the wide variety of strains of S. pyogenes present in the environment and the large amount of time and number of people that will be needed for appropriate trials for safety and efficacy of the vaccine.[60][61]
Treatment

The treatment of choice is penicillin, and the duration of treatment is around 10 days.[62] Antibiotic therapy (using injected penicillin) has been shown to reduce the risk of acute rheumatic fever.[63] In individuals with a penicillin allergy, erythromycin, other macrolides, and cephalosporins have been shown to be effective treatments.[64]
Treatment with amoxicillin/clavulanic acid, or clindamycin is appropriate if deep oropharyngeal abscesses are present, in conjunction with aspiration or drainage. In cases of streptococcal toxic shock syndrome, treatment consists of penicillin and clindamycin, given with intravenous immunoglobulin.[65][66]
For toxic shock syndrome and necrotizing fasciitis, high-dose penicillin and clindamycin are used. Additionally, for necrotizing fasciitis, surgery is often needed to remove damaged tissue and stop the spread of the infection.[59]
No instance of penicillin resistance has been reported to date, although since 1985, many reports of penicillin tolerance have been made.[67] The reason for the failure of penicillin to treat S. pyogenes is most commonly patient noncompliance, but in cases where patients have been compliant with their antibiotic regimen, and treatment failure still occurs, another course of antibiotic treatment with cephalosporins is common.[64]
The 30-valent N-terminal M-protein-based vaccine as well as the M-protein vaccine (minimal epitope J8 vaccine) are two vaccines for GAS that are currently getting close or becoming clinical studies, however, other vaccines using conserved epitopes are progressing. [68]
Public health policies internationally reflect differing views of how the close contacts of people affected by severe Group A streptococcal infections should be treated. Health Canada[69] and the US CDC recommend close contacts see their doctor for full evaluation and may require antibiotics;[70] current UK Health Protection Agency guidance is that, for a number of reasons, close contacts should not receive antibiotics unless they are symptomatic but that they should receive information and advice to seek immediate medical attention if they develop symptoms.[51] However, guidance is clearer in the case of mother-baby pairs: both mother and baby should be treated if either develops an invasive GAS infection within the first 28 days following birth[51] (though some evidence suggests that this guidance is not routinely followed in the UK[71]).
Epidemiology
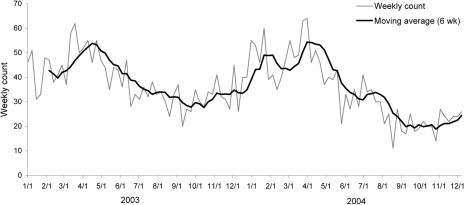
Environmental factors, such as less crowding and the increase of family living space, can account for the reduction in incidence and severity of group A streptococci.[1] [72] With more space for individuals to reside in, it provides the bacteria with less opportunities to spread from person to person. This is especially important considering an estimated 500,000 deaths worldwide all occurring after acute rheumatic fever, invasive infection, or subsequent heart disease can be accredited to GAS.[73] This number is quite large, often leaving the health care system encumbered, since 91 percent of patients infected with invasive GAS need to be hospitalized with 8950-11,500 episodes and 1050-1850 deaths taking place each year. [73] A later study that occurred from 2005-2012 found that there were 10,649-13,434 cases consequently resulting in 1136-1607 deaths per year. [68]
GAS causes 15–30% of the childhood cases and 10% of adult cases of Strep throat.[7]
History

Cases of GAS were evident before World War I. This was shown by a training camp located in Texas, where a harmful strain of pneumonia complicating measles was caused by a strain of Streptococcus.[72] Existence of streptococci strains was additionally found in World War II. An epidemic of streptococcal infection in the United States Navy during this war indicated that this type of disease was able to exist and spread in formerly unexposed individuals by environments that serological types of group A streptococci preferred. [72] In later years, a positive test result for the presence of group A streptococci was found in 32.1 percent of individuals after throat cultures were carried out in a 20 yearlong (1953/1954-1973/1974) study performed in Nashville, TN. [72] Also, from 1972-1974, recurring GAS illness was observed with a prevalence of 19 percent in school-aged children as well as a prevalence rate of 25 percent in families. [72] The severity of streptococcal infections has decreased over the years, and so has rheumatic fever (a sequelae of GAS) which is indicated by the change in numerous hospitals from containing wards allocated for the sole purpose of treating rheumatic fever to hardly seeing the disease at all. [72]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 1.32 1.33 1.34 1.35 1.36 1.37 1.38 1.39 1.40 1.41 1.42 1.43 1.44 1.45 1.46 1.47 1.48 1.49 Stevens, Dennis L.; Bryant, Amy E.; Hagman, Melissa M. (2020). "274. Nonpneumococcal streptococcal infections and rheumatic fever". In Goldman, Lee; Schafer, Andrew I. (eds.). Goldman-Cecil Medicine. Vol. 2 (26th ed.). Philadelphia: Elsevier. pp. 1871–1878. ISBN 978-0-323-55087-1.
- ↑ 2.0 2.1 2.2 2.3 2.4 Newberger, Ryan; Gupta, Vikas (2022). "Streptococcus Group A". StatPearls. StatPearls Publishing. Archived from the original on 17 November 2020. Retrieved 7 January 2023.
- ↑ 3.0 3.1 3.2 Khan, Zartash Zafar. "Group A Streptococcal (GAS) Infections Workup: Approach Considerations, Pharyngitis, Acute Rheumatic Fever". eMedicine. Archived from the original on 24 December 2022. Retrieved 13 January 2023.
- ↑ 4.0 4.1 4.2 "Pharyngitis (Strep Throat): Information For Clinicians | CDC". www.cdc.gov. 19 December 2022. Archived from the original on 27 October 2020. Retrieved 14 January 2023.
- ↑ 5.0 5.1 "Amoxicillin Shortage: Antibiotic Options for Common Pediatric Conditions". www.aap.org. American Academy of Paediatrics. 21 November 2022. Archived from the original on 1 January 2023. Retrieved 14 January 2023.
- ↑ Khan, Zartash Zafar (17 October 2021). "Group A Streptococcal (GAS) Infections: Etiology". eMedicine. Archived from the original on 22 February 2011. Retrieved 6 January 2023.
- ↑ 7.0 7.1 7.2 Cohen-Poradosu, Ronit; Dennis L. Kasper (2007). "Group A Streptococcus Epidemiology and Vaccine Implications". Clinical Infectious Diseases. 45 (7): 863–5. doi:10.1086/521263. PMID 17806050.
- ↑ Carapetis, JR; Steer, AC; Mulholland, EK; Weber, M (November 2005). "The global burden of group A streptococcal diseases". The Lancet Infectious Diseases. 5 (11): 685–94. doi:10.1016/S1473-3099(05)70267-X. PMID 16253886.
- ↑ Ferretti JJ, Stevens DL, Fischetti VA, editors (2016). Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. Oklahoma City, OK: University of Oklahoma Health Sciences Center. PMID 26866208. Archived from the original on 2019-09-19. Retrieved 2022-03-05.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ James, William D.; Elston, Dirk; Treat, James R.; Rosenbach, Misha A.; Neuhaus, Isaac (2020). "14. Bacterial infections". Andrews' Diseases of the Skin: Clinical Dermatology (13th ed.). Edinburgh: Elsevier. p. 259-263. ISBN 978-0-323-54753-6. Archived from the original on 2023-01-15. Retrieved 2023-01-13.
- ↑ Group A streptococcal infection at National Institutes of Health
- ↑ 12.0 12.1 12.2 "Streptococcal Infections (Invasive Group A Srtep)". New York City Department of Health a. Archived from the original on 6 November 2012. Retrieved 21 November 2012.
- ↑ Gamba MA, Martinelli M, Schaad HJ, Streuli RA, DiPersio J, Matter L, et al. (1997). "Familial transmission of a serious disease producing group A streptococcus clone:case reports and review". Clin Infect Dis. 24 (6): 1118–21. doi:10.1086/513636. PMID 9195067.
- ↑ Al-Khadidi, Fawzyh J.; AlSheheri, Mohammed A.; AlFawaz, Tariq S.; Enani, Mushira A.; AlAqeel, Abdulaziz A.; AlShahrani, Dayel A. (October 2017). "Group A Streptococcal bacteraemia: Experience at King Fahad Medical City in Riyadh, Saudi Arabia". Saudi Medical Journal. 38 (10): 1034–1037. doi:10.15537/smj.2017.10.20966. Archived from the original on 12 January 2023. Retrieved 10 January 2023.
- ↑ Dubost, Jean-Jacques; Soubrier, Martin; De Champs, Christophe; Ristori, Jean-Michel; Sauvezie, Bernard (July 2004). "Streptococcal septic arthritis in adults. A study of 55 cases with a literature review". Joint Bone Spine. 71 (4): 303–311. doi:10.1016/S1297-319X(03)00122-2. ISSN 1297-319X. Archived from the original on 2022-06-16. Retrieved 2023-01-10.
- ↑ Seng, P.; Vernier, M.; Gay, A.; Pinelli, P.-O.; Legré, R.; Stein, A. (July 2016). "Clinical features and outcome of bone and joint infections with streptococcal involvement: 5-year experience of interregional reference centres in the south of France". New Microbes and New Infections. 12: 8–17. doi:10.1016/j.nmni.2016.03.009. Archived from the original on 12 January 2023. Retrieved 10 January 2023.
- ↑ de Almeida Torres, Rosângela Stadnick Lauth; Fedalto, Luiz Ernesto; de Almeida Torres, Rômulo Francisco; Steer, Andrew C.; Smeesters, Pierre Robert (February 2013). "Group A Streptococcus Meningitis in Children". The Pediatric Infectious Disease Journal. 32 (2): 110. doi:10.1097/INF.0b013e31826fd4af. ISSN 0891-3668. Archived from the original on 12 January 2023. Retrieved 10 January 2023.
- ↑ Del Mar, Chris (1 August 2016). "Acute sinusitis and sore throat in primary care". Australian Prescriber. 39 (4): 116–118. doi:10.18773/austprescr.2016.046. Archived from the original on 3 February 2022. Retrieved 10 January 2023.
- ↑ Parra-Herran, Carlos (2019). "8. Soft tissue lesions of the vulva and vagina". In Zheng, Wenxin; Fadare, Oluwole; Quick, Charles Matthew; Shen, Danhua; Guo, Donghui (eds.). Gynecologic and Obstetric Pathology. Vol. 1. Singapore: Springer. p. 233. ISBN 978-981-13-3015-5. Archived from the original on 2023-01-17. Retrieved 2023-01-16.
- ↑ Muller, Matthew P.; Low, Donald E.; Green, Karen A.; Simor, Andrew E.; Loeb, Mark; Gregson, Daniel; McGeer, Allison (24 February 2003). "Clinical and Epidemiologic Features of Group A Streptococcal Pneumonia in Ontario, Canada". Archives of Internal Medicine. 163 (4): 467–472. doi:10.1001/archinte.163.4.467. ISSN 0003-9926. Archived from the original on 15 June 2022. Retrieved 10 January 2023.
- ↑ Michaels, Marian `G.; Williams, John V. (2023). "13. Infectious diseases". In Zitelli, Basil J.; McIntire, Sara C.; Nowalk, Andrew J.; Garrison, Jessica (eds.). Zitelli and Davis' Atlas of Pediatric Physical Diagnosis (8th ed.). Philadelphia: Elsevier. pp. 468–469. ISBN 978-0-323-77788-9. Archived from the original on 17 December 2022. Retrieved 17 December 2022.
- ↑ 22.0 22.1 22.2 22.3 22.4 Stevens, Dennis L.; Bryant, Amy E. (2022). "21. Life-threatening skin and soft tissue infections". In Jong, Elaine C.; Stevens, Dennis L. (eds.). Netter's Infectious Diseases (2nd ed.). Elsevier. pp. 94–95. ISBN 978-0-323-71159-3. Archived from the original on 2023-01-12. Retrieved 2023-01-11.
- ↑ Snelling, Thomas L; Carapetis, Jonathan R (2013). "Group A Streptococcus". Hunter's Tropical Medicine and Emerging Infectious Disease: 391–401. doi:10.1016/B978-1-4160-4390-4.00036-9. Archived from the original on 10 January 2023. Retrieved 9 January 2023.
- ↑ Jim Dwyer (July 11, 2012). "An Infection, Unnoticed, Turns Unstoppable". The New York Times. Archived from the original on April 25, 2019. Retrieved July 12, 2012.
- ↑ Jim Dwyer (July 18, 2012). "After Boy's Death, Hospital Alters Discharging Procedures". The New York Times. Archived from the original on September 22, 2019. Retrieved July 19, 2012.
- ↑ Long, Sarah S.; Pickering, Larry K.; Prober, Charles G. (1 January 2012). Principles and Practice of Pediatric Infectious Disease. Elsevier Health Sciences. p. 700. ISBN 978-1-4377-2702-9. Archived from the original on 15 January 2023. Retrieved 14 January 2023.
- ↑ "Acute Rheumatic Fever: Information For Clinicians | CDC". www.cdc.gov. 7 October 2022. Archived from the original on 13 December 2022. Retrieved 8 January 2023.
- ↑ 28.0 28.1 Karthikeyan, Ganesan; Guilherme, Luiza (14 July 2018). "Acute rheumatic fever". The Lancet. 392 (10142): 161–174. doi:10.1016/S0140-6736(18)30999-1. ISSN 0140-6736. Archived from the original on 29 August 2021. Retrieved 12 January 2023.
- ↑ Sika-Paotonu, D.; Beaton, A.; Raghu, A.; Steer, A.; Carapetis, J. (2016). "Acute Rheumatic Fever and Rheumatic Heart Disease". Streptococcus pyogenes : Basic Biology to Clinical Manifestations. Oklahoma City: University of Oklahoma Health Sciences Center. PMID 28379675. Archived from the original on 2022-02-10. Retrieved 2022-03-05.
- ↑ LeMone, Priscilla; Burke, Karen; Dwyer, Trudy; Levett-Jones, Tracy; Moxham, Lorna; Reid-Searl, Kerry (20 May 2015). Medical-Surgical Nursing. Pearson Higher Education AU. p. 1059. ISBN 978-1-4860-1440-8. Retrieved 8 January 2023.
- ↑ Coelho Mota, Cleonice C.; Aiello, Vera Demarchi; Anderson, Robert H. (1 January 2010). "CHAPTER 54B - Chronic Rheumatic Heart Disease". Paediatric Cardiology (Third Edition). Churchill Livingstone. pp. 1115–1134. ISBN 978-0-7020-3064-2. Retrieved 11 January 2023.
- ↑ Kumar, Vinay; Abbas, Abul K.; Aster, Jon C. (8 March 2017). Robbins Basic Pathology E-Book. Elsevier Health Sciences. ISBN 978-0-323-39413-0. Archived from the original on 12 January 2023. Retrieved 12 January 2023.
- ↑ Majmundar, Vidit D.; Nagalli, Shivaraj (2022). "Erythema Marginatum". StatPearls. StatPearls Publishing. Archived from the original on 23 October 2022. Retrieved 8 January 2023.
- ↑ "Sydenham Chorea | National Institute of Neurological Disorders and Stroke". www.ninds.nih.gov. Archived from the original on 8 January 2023. Retrieved 8 January 2023.
- ↑ Rowland, Lewis P.; Pedley, Timothy A. (2010). Merritt's Neurology. Lippincott Williams & Wilkins. p. 1188. ISBN 978-0-7817-9186-1. Archived from the original on 2023-01-12. Retrieved 2023-01-11.
- ↑ Sika-Paotonu, Dianne; Beaton, Andrea; Raghu, Aparna; Steer, Andrew; Carapetis, Jonathan (2016). "Acute Rheumatic Fever and Rheumatic Heart Disease". Streptococcus pyogenes: Basic Biology to Clinical Manifestations. University of Oklahoma Health Sciences Center. Archived from the original on 10 February 2022. Retrieved 11 January 2023.
- ↑ "Post-Streptococcal Glomerulonephritis: For Clinicians | CDC". www.cdc.gov. 27 June 2022. Archived from the original on 13 December 2022. Retrieved 8 January 2023.
- ↑ "Poststreptococcal glomerulonephritis (GN): MedlinePlus Medical Encyclopedia". medlineplus.gov. Archived from the original on 8 January 2023. Retrieved 8 January 2023.
- ↑ 39.0 39.1 Dale RC (December 2017). "Tics and Tourette: a clinical, pathophysiological and etiological review". Curr Opin Pediatr (Review). 29 (6): 665–673. doi:10.1097/MOP.0000000000000546. PMID 28915150. S2CID 13654194.
- ↑ 40.0 40.1 40.2 40.3 Marazziti D, Mucci F, Fontenelle LF (July 2018). "Immune system and obsessive-compulsive disorder". Psychoneuroendocrinology (Review). 93: 39–44. doi:10.1016/j.psyneuen.2018.04.013. PMID 29689421. S2CID 13681480.
- ↑ 41.0 41.1 41.2 41.3 Zibordi F, Zorzi G, Carecchio M, Nardocci N (March 2018). "CANS: Childhood acute neuropsychiatric syndromes". Eur J Paediatr Neurol (Review). 22 (2): 316–320. doi:10.1016/j.ejpn.2018.01.011. PMID 29398245.
- ↑ Wilbur C, Bitnun A, Kronenberg S, Laxer RM, Levy DM, Logan WJ, Shouldice M, Yeh EA (May 2019). "PANDAS/PANS in childhood: Controversies and evidence". Paediatr Child Health. 24 (2): 85–91. doi:10.1093/pch/pxy145. PMC 6462125. PMID 30996598.
- ↑ Sigra S, Hesselmark E, Bejerot S (March 2018). "Treatment of PANDAS and PANS: a systematic review". Neurosci Biobehav Rev. 86: 51–65. doi:10.1016/j.neubiorev.2018.01.001. PMID 29309797. S2CID 40827012. Archived from the original on 2022-01-11. Retrieved 2022-03-05.
- ↑ Tanaka D, Isobe J, Watahiki M, Nagai Y, Katsukawa C, Kawahara R, Endoh M, Okuno R, Kumagai N, Matsumoto M, Morikawa Y, Ikebe T, Watanabe H (2008). "Genetic features of clinical isolates of Streptococcus dysgalactiae subsp. equisimilis possessing Lancefield's group A antigen". J. Clin. Microbiol. 46 (4): 1526–9. doi:10.1128/JCM.02188-07. PMC 2292899. PMID 18305132.
- ↑ "Group A Streptococcal (GAS) Disease". Centers for Disease Control and Prevention. Archived from the original on December 19, 2007. Retrieved November 21, 2012.
- ↑ Bryant, Amy E.; Stevens, Dennis L. (1 January 2015). "199 - Streptococcus pyogenes". Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (Eighth Edition). W.B. Saunders. pp. 2285–2299.e4. ISBN 978-1-4557-4801-3. Archived from the original on 13 December 2020. Retrieved 14 January 2023.
- ↑ Patterson, Maria Jevitz (1996). "Streptococcus". Medical Microbiology (4th ed.). University of Texas Medical Branch at Galveston. ISBN 978-0-9631172-1-2. Archived from the original on 2009-04-25. Retrieved 14 January 2023.
- ↑ "Streptococcus pyogenes - Pathogen Safety Data Sheets". Government of Canada, Public Health Agency of Canada. 2001-09-26. Archived from the original on 2017-01-17. Retrieved 2023-01-06.
- ↑ Wierzbicki, I. H.; Campeau, A.; Dehaini, D.; Holay, M.; Wei, X.; Greene, T.; Ying, M.; Sands, J. S.; Lamsa, A.; Zuniga, E.; Pogliano, K.; Fang, R. H.; Larock, C. N.; Zhang, L.; Gonzalez, D. J. (2019). "Group A Streptococcal S Protein Utilizes Red Blood Cells as Immune Camouflage and Is a Critical Determinant for Immune Evasion". Cell Reports. 29 (10): 2979–2989.e15. doi:10.1016/j.celrep.2019.11.001. PMC 6951797. PMID 31801066.
- ↑ "Streptococcal Infections: What is Group A Strepotococcus (GAS)". Archived from the original on 15 July 2017. Retrieved 21 November 2012.
- ↑ 51.0 51.1 51.2 Health Protection Agency, Group A Streptococcus Working Group (2004). "Interim UK guidelines for management of close community contacts of invasive group A streptococcal disease" (PDF). Commun Dis Public Health. 7 (4): 354–61. PMID 15786581. Archived from the original (PDF) on 2008-06-25. Retrieved 2008-05-09.
- ↑ Australia, Healthdirect (9 September 2021). "Group A Streptococcal disease". www.healthdirect.gov.au. Archived from the original on 8 December 2022. Retrieved 9 January 2023.
- ↑ Stevens, Dennis L.; Bryant, Amy E. (2016). "Severe Group A Streptococcal Infections". Streptococcus pyogenes: Basic Biology to Clinical Manifestations. University of Oklahoma Health Sciences Center. Archived from the original on 6 October 2022. Retrieved 9 January 2023.
- ↑ Nguyen, Michelle; Bendi, Venkata Sunil; Guduru, Mounika; Olson, Evan; Vivekanandan, Renuga; Foral, Pamela A; Velagapudi, Manasa (22 August 2018). "Postpartum Invasive Group A Streptococcus Infection: Case Report and Mini-review". Cureus. doi:10.7759/cureus.3184. Archived from the original on 14 December 2020. Retrieved 12 January 2023.
- ↑ 55.0 55.1 Cunningham, M. W. (July 2000). "Pathogenesis of group A streptococcal infections". Clinical Microbiology Reviews. 13 (3): 470–511. doi:10.1128/CMR.13.3.470. ISSN 0893-8512. PMC 88944. PMID 10885988.
- ↑ Kellogg JA, Bankert DA, Elder CJ, Gibbs JL, Smith MC (September 2001). "Identification of Streptococcus pneumoniae revisited". J. Clin. Microbiol. 39 (9): 3373–5. doi:10.1128/jcm.39.9.3373-3375.2001. PMC 88350. PMID 11526182.
- ↑ Burdash NM, West ME (March 1982). "Identification of Streptococcus pneumoniae by the Phadebact coagglutination test". J. Clin. Microbiol. 15 (3): 391–4. doi:10.1128/JCM.15.3.391-394.1982. PMC 272105. PMID 7076811.
- ↑ "Pyrrolidonyl Arylamidase (PYR) Test: Principle, procedure and results—microbeonline". 12 November 2013. Archived from the original on 7 April 2019. Retrieved 5 March 2022.
- ↑ 59.0 59.1 "Group A Strep". CDC.gov. CDC. Archived from the original on 30 October 2019. Retrieved 7 December 2014.
- ↑ 60.0 60.1 Good MF, Batzloff MR, Pandey M (November 2013). "Strategies in the development of vaccines to prevent infections with group A streptococcus". Human Vaccines & Immunotherapeutics. 9 (11): 2393–7. doi:10.4161/hv.25506. PMC 3981849. PMID 23863455.
- ↑ "Initiative for Vaccine Research (IVR) – Group A Streptococcus". World Health Organization. Archived from the original on March 22, 2006. Retrieved 15 June 2012.
- ↑ Falagas ME, Vouloumanou EK, Matthaiou DK, Kapaskelis AM, Karageorgopoulos DE (2008). "Effectiveness and safety of short-course vs long-course antibiotic therapy for group a beta hemolytic streptococcal tonsillopharyngitis: a meta-analysis of randomized trials". Mayo Clin Proc. 83 (8): 880–9. doi:10.4065/83.8.880. PMID 18674472.
- ↑ HOUSER HB, WANNAMAKER LW, RAMMELKAMP CH, DENNY FW, BRINK WR, HAHN EO, DINGLE JH (1950). "Prophylaxis of acute rheumatic fever by treatment of the preceding streptococcal infection with various amounts of penicillin". J Lab Clin Med. 36 (5): 839. PMID 14784714.
- ↑ 64.0 64.1 Khan, Zartash. "Group A Streptococcal Infections Treatment & Management". Medscape. Archived from the original on 7 October 2017. Retrieved 7 December 2014.
- ↑ Schroeder, Barrett M. (15 February 2003). "Diagnosis and Management of Group A Streptococcal Pharyngitis". American Family Physician. pp. 880–884. Archived from the original on 8 January 2023. Retrieved 8 January 2023.
- ↑ "Streptococcal Toxic Shock Syndrome: For Clinicians | CDC". www.cdc.gov. 27 June 2022. Archived from the original on 23 December 2022. Retrieved 11 January 2023.
- ↑ Kim KS, Kaplan EL (1985). "Association of penicillin tolerance with failure to eradicate group A streptococci from patients with pharyngitis". J Pediatr. 107 (5): 681–4. doi:10.1016/S0022-3476(85)80392-9. PMID 3903089.
- ↑ 68.0 68.1 Nelson, George E.; Pondo, Tracy; Toews, Karrie-Ann; Farley, Monica M.; Lindegren, Mary Lou; Lynfield, Ruth; Aragon, Deborah; Zansky, Shelley M.; Watt, James P.; Cieslak, Paul R.; Angeles, Kathy (2016-08-15). "Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005–2012". Clinical Infectious Diseases. 63 (4): 478–486. doi:10.1093/cid/ciw248. ISSN 1058-4838. PMC 5776658. PMID 27105747.
- ↑ Guidelines for management of contacts of cases of invasive group A streptococcal disease (GAS) including streptococcal toxic shock syndrome (STSS) and necrotising fasciitis. Toronto, Ontario: Ministry of Health; 1995. Available at: [1]
- ↑ "Disease Listing, Group a Streptococcal, General Info | CDC Bacterial, Mycotic Diseases". Archived from the original on 2007-12-19. Retrieved 2007-12-11.
- ↑ Howard, SJ; Stoker, K; Foster, K (16 June 2015). "Public health management of group A streptococcal infection in mother-baby pairs in England; a case series review". Antimicrobial Resistance and Infection Control. 4 (Suppl 1): P107. doi:10.1186/2047-2994-4-S1-P107. PMC 4474978.
- ↑ 72.0 72.1 72.2 72.3 72.4 72.5 Quinn, Robert W. (1982), "Streptococcal Infections", Bacterial Infections of Humans, Springer US, pp. 525–552, doi:10.1007/978-1-4757-1140-0_29, ISBN 9781475711424
- ↑ 73.0 73.1 O'Loughlin, R. E.; Roberson, A.; Cieslak, P. R.; Lynfield, R.; Gershman, K.; Craig, A.; Albanese, B. A.; Farley, M. M.; Barrett, N. L.; Spina, N. L.; Beall, B. (2007-10-01). "The Epidemiology of Invasive Group A Streptococcal Infection and Potential Vaccine Implications: United States, 2000-2004". Clinical Infectious Diseases. 45 (7): 853–862. doi:10.1086/521264. ISSN 1058-4838. PMID 17806049.
Note: Elements of the original text of this article are taken from the NIH Fact Sheet "Group A Streptococcal Infections", dated March 1999. As a work of the U.S. Federal Government without any other copyright notice, this is assumed to be a public domain resource.
External links
| Classification | |
|---|---|
| External resources |