Glatiramer acetate
 | |
| Names | |
|---|---|
| Trade names | Copaxone,[1] Glatopa,[2] Brabio, others |
| Other names | Glatiramer acetate, copolymer 1, Cop-1 |
| Clinical data | |
| Drug class | Peptides[3] |
| Main uses | Multiple sclerosis[4] |
| Side effects | Pain at site of injection, rash, shortness of breath, chest pain[1] |
| Pregnancy category |
|
| Routes of use | Subcutaneous injection |
| Typical dose | 20 mg OD[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603016 |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C25H45N5O13 |
| Molar mass | 623.657 g·mol−1 |
Glatiramer acetate, sold under the brand name Copaxone among others, is a medication used to treat multiple sclerosis.[4] Specifically it is used for relapsing forms of MS including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.[1] It is given by injection under the skin.[4]
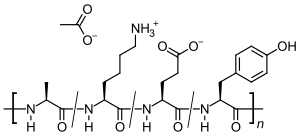
Common side effects include pain at the site of injection, rash, shortness of breath, and chest pain.[1] Other side effects may include flushing, anxiety, lipoatrophy, and liver problems.[1] While there is no clear harm with use in pregnancy, such use has not been well studied.[7] It is a mixture of different sized peptides that are composed of the four amino acids, namely glutamic acid, lysine, alanine, and tyrosine.[3] It is believed to be similar to myelin basic protein (MBP) and inactivate antibodies directed against it.[3]
Glatiramer acetate was approved for medical use in the United States in 1996.[4] It is on the World Health Organization's List of Essential Medicines.[8] In the United Kingdom 4 weeks of medication costs the NHS about £515 as of 2021.[9] In the United States this amount costs about 1,200 USD.[10]
Medical uses
Glatiramer acetate is used to treat relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.[1]
A 2010 Cochrane review concluded that glatiramer acetate had partial efficacy in "relapse-related clinical outcomes" but no effect on progression of the disease.[11] As a result, it is approved by the FDA for reducing the frequency of relapses, but not for reducing the progression of disability.[1][2]
A 15-year followup of the original trial compared patients who continued with glatiramer to patients who dropped out of the trial. Patients with glatiramer had reduced relapse rates, and decreased disability progression and transition to secondary progressive MS, compared to patients who did not continue glatiramer. However, the two groups were not necessarily comparable, as it was no longer a randomized trial. There were no long-term safety issues.[12]
Dosage
It may be used at a dose of 20 mg per day or 40 mg three times per week.[1]
Side effects
Side effects may include a lump at the injection site (injection site reaction) in approximately 30% of users, and aches, fever, chills (flu-like symptoms) in approximately 10% of users.[13] Side effect symptoms are generally mild in nature. A reaction that involves flushing, shortness of breath, anxiety and rapid heartbeat has been reported soon after injection in up to 5% of patients (usually after inadvertently injecting directly into a vein). These side effects subside within thirty minutes. Over time, a visible dent at a repeat-injection site can occur due to the local destruction of fat tissue, known as lipoatrophy, that may develop.
More serious side effects have been reported for glatiramer acetate, according to the FDA's prescribing label, these include serious side effects to the cardiovascular, digestive (including the liver), hematopoietic, lymphatic, musculoskeletal, nervous, respiratory, and urogenital systems as well as special senses (in particular the eyes). Metabolic and nutritional disorders have also been reported; however a link between glatiramer acetate and these adverse effects has not been established.[1][2]
It may also cause Jessner lymphocytic infiltrate.[14]
-
Nicolau syndrome associated subcutaneous glatiramer acetate injection[15] a) Nicolau Syndrome on left abdomen b) erythematous, purpuric and haemorrhagic patch, at site of subcutaneous glatiramer acetate c) 3 weeks after GA injection livedoid patch disappeared
-
An injection site reaction on the upper left arm
Mechanism of action
Glatiramer acetate is a random polymer (average molecular mass 6.4 kD) composed of four amino acids found in myelin basic protein. The mechanism of action for glatiramer acetate is not fully elucidated. It is thought to act by modifying immune processes that are believed to be responsible for the pathogenesis of MS. Administration of glatiramer acetate shifts the population of T cells from proinflammatory Th1 T-cells to regulatory Th2 T-cells that suppress the inflammatory response.[16] Given its resemblance to myelin basic protein, glatiramer acetate may act as a decoy, diverting an autoimmune response against myelin. This hypothesis is supported by findings of studies that have been carried out to explore the pathogenesis of experimental autoimmune encephalomyelitis (EAE), a condition induced in several animal species through immunization against central nervous system derived material containing myelin and often used as an experimental animal model of MS. Studies in animals and in vitro systems suggest that upon its administration, glatiramer acetate-specific regulatory T cells (Tregs) are induced and activated in the periphery, inhibiting the inflammatory reaction to myelin basic protein.[1][2]
The integrity of the blood-brain barrier, however, is not appreciably affected by glatiramer acetate, at least not in the early stages of treatment. Glatiramer acetate has been shown in clinical trials to reduce the number and severity of multiple sclerosis exacerbations.[17]
History
Glatiramer acetate was originally discovered at the Weizmann Institute of Science. Three main clinical trials followed to demonstrate safety and efficacy: The first trial was performed in a single center, double-blind, placebo controlled trial and included 50 patients.[18] The second trial was a two-year, multi-center, randomized, double-blind, placebo controlled trial and involved 251 patients.[19] The third trial was a double-blind MRI study involving participation of 239 patients.[20]
Society and culture
Marketing
Glatiramer acetate has been approved for marketing in numerous countries worldwide, including the United States, Israel, Canada and 24 European Union countries.[21][22] Approval in the U.S. was obtained in 1997.[23] Glatiramer acetate was approved for marketing in the U.K. in August 2000, and launched in December.[24] This first approval in a major European market led to approval across the European Union under the mutual recognition procedure. Iran is proceeding with domestic manufacture of glatiramer acetate.[25][26]
Patent status
Novartis subsidiary Sandoz has marketed Glatopa since 2015, a generic version of the original Teva 20 mg formulation that requires daily injection.[27]
Teva developed a long-acting 40 mg formulation, marketed since 2015, which reduced required injections to three per week.[28] In October 2017, the FDA approved a generic version, which is manufactured in India by Natco Pharma, and imported and sold by Dutch firm Mylan.[29][30] In February 2018, Sandoz received FDA approval for their generic version.[31] In parallel with the development and approval processes, the generic competitors have disputed Teva's newer patents, any of which if upheld, would prevent marketing of long-acting generics.[32]
While the patent on the chemical drug expired in 2015,[33] Teva obtained new US patents covering pharmaceutical formulations for long-acting delivery.[34] Litigation from industry competitors in 2016-2017 resulted in the new patents being judged invalid.[35][36] In October 2018, the U.S. Court of Appeals for the Federal Circuit upheld the patent invalidation for obviousness.[37][38] The case reflects the larger controversy over evergreening of generic drugs.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 "Copaxone- glatiramer acetate injection, solution". DailyMed. 23 July 2020. Archived from the original on 31 October 2021. Retrieved 11 November 2020.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Glatopa- glatiramer acetate injection, solution". DailyMed. 31 July 2020. Archived from the original on 17 November 2020. Retrieved 11 November 2020.
- ↑ 3.0 3.1 3.2 Babaesfahani, A; Bajaj, T (January 2021). "Glatiramer". StatPearls. PMID 31082051.
- ↑ 4.0 4.1 4.2 4.3 "Glatiramer Monograph for Professionals". Drugs.com. Archived from the original on 27 November 2021. Retrieved 3 December 2021.
- ↑ "Brabio 20 mg/mL Solution for Injection, Pre-filled Syringe - Summary of Product Characteristics (SmPC)". (emc). Archived from the original on 17 November 2020. Retrieved 11 November 2020.
- ↑ "Copaxone 20 mg/ml solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC)". (emc). 29 September 2020. Archived from the original on 12 November 2020. Retrieved 11 November 2020.
- ↑ "Glatiramer Use During Pregnancy". Drugs.com. Archived from the original on 3 December 2020. Retrieved 3 December 2021.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 898. ISBN 978-0857114105.
- ↑ "Glatiramer Prices and Glatiramer Coupons - GoodRx". GoodRx. Retrieved 3 December 2021.
- ↑ La Mantia L, Munari LM, Lovati R (May 2010). "Glatiramer acetate for multiple sclerosis". The Cochrane Database of Systematic Reviews. 5 (5): CD004678. doi:10.1002/14651858.CD004678.pub2. PMID 20464733.
- ↑ Ford C, Goodman AD, Johnson K, Kachuck N, Lindsey JW, Lisak R, et al. (March 2010). "Continuous long-term immunomodulatory therapy in relapsing multiple sclerosis: results from the 15-year analysis of the US prospective open-label study of glatiramer acetate". Multiple Sclerosis. 16 (3): 342–50. doi:10.1177/1352458509358088. PMC 2850588. PMID 20106943.
- ↑ "Copaxone". MediGuard. Archived from the original on 2018-02-03. Retrieved 2021-04-15.
- ↑ Krafchik BR (2011). "Reaction Patterns". In Schachner LA, Hansen RC (eds.). Pediatric Dermatology. Elsevier Health Sciences. p. 1022. ISBN 978-0-7234-3665-2. Archived from the original on 2021-08-28. Retrieved 2021-04-15.
- ↑ Ciprian, Sandro; Lava, Sebastiano A. G.; Milani, Gregorio P.; Bianchetti, Mario G.; Consolascio, Danilo; Lardelli, Pietro F. (2 November 2021). "Nicolau syndrome caused by Glatiramer". Multiple Sclerosis and Related Disorders: 103365. doi:10.1016/j.msard.2021.103365. ISSN 2211-0348. Archived from the original on 11 January 2022. Retrieved 8 January 2022.
- ↑ Arnon R, Sela M (1999). "The chemistry of the Copaxone drug" (PDF). Chem. Israel. 1: 12–17. Archived from the original (PDF) on 2003-09-07.
- ↑ "Copaxone". All About Multiple Sclerosis. Archived from the original on 2020-11-14. Retrieved 2021-04-15.
- ↑ Bornstein MB, Miller A, Slagle S, Weitzman M, Crystal H, Drexler E, Keilson M, Merriam A, Wassertheil-Smoller S, Spada V (August 1987). "A pilot trial of Cop 1 in exacerbating-remitting multiple sclerosis". The New England Journal of Medicine. 317 (7): 408–14. doi:10.1056/NEJM198708133170703. PMID 3302705.
- ↑ Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, Myers LW, Panitch HS, Rose JW, Schiffer RB (July 1995). "Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group". Neurology. 45 (7): 1268–76. doi:10.1212/WNL.45.7.1268. PMID 7617181. S2CID 28895177.
- ↑ Comi G, Filippi M, Wolinsky JS (March 2001). "European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging--measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group". Annals of Neurology. 49 (3): 290–7. doi:10.1002/ana.64. PMID 11261502. S2CID 35614752.
- ↑ McKeage K (May 2015). "Glatiramer Acetate 40 mg/mL in Relapsing-Remitting Multiple Sclerosis: A Review". CNS Drugs. 29 (5): 425–32. doi:10.1007/s40263-015-0245-z. PMID 25906331. S2CID 30186027.
- ↑ Comi G, Amato MP, Bertolotto A, Centonze D, De Stefano N, Farina C, et al. (2016). "The heritage of glatiramer acetate and its use in multiple sclerosis". Multiple Sclerosis and Demyelinating Disorders. 1 (1). doi:10.1186/s40893-016-0010-2.
- ↑ "Copaxone". CenterWatch. Archived from the original on 2019-08-05. Retrieved 2021-04-15.
- ↑ "Teva's Copaxone approved in UK". The Pharma Letter. Archived from the original on 2021-10-31. Retrieved 2021-04-15.
- ↑ "Glatiramer Acetate". Tofigh Daru Research and Engineering Company. Archived from the original on 2018-10-21. Retrieved 2021-04-15.
- ↑ Isayev S, Jafarov T (1 May 2012). "Iran to manufacture multiple sclerosis cure". Trend News Agency. Archived from the original on 1 December 2017. Retrieved 15 April 2021.
- ↑ "Sandoz announces US launch of Glatopa". Novartis. 2015. Archived from the original on 2018-02-03. Retrieved 2021-04-15.
- ↑ Silva P (9 October 2015). "New 3-Times-Per-Week Regimen For Teva's Copaxone". Multiple Sclerosis News Today. Archived from the original on 24 February 2019. Retrieved 15 April 2021.
- ↑ Erman M, Grover D (3 October 2017). "Mylan surges, Teva slumps after FDA okays Copaxone copy". Reuters. Archived from the original on 4 October 2017. Retrieved 4 October 2017.
- ↑ "NATCO's marketing partner Mylan receives final approval of generic glatiramer acetate, for both 20 mg/mL and 40 mg/mL versions". NATCO Pharma (India). 3 October 2017. Archived from the original on 10 January 2019. Retrieved 4 October 2017.>
- ↑ "Sandoz announces US FDA approval and launch of Glatopa 40 mg/mL". Novartis International AG. February 13, 2018. Archived from the original on May 11, 2018. Retrieved May 10, 2018.
- ↑ "Teva's Copaxone still growing despite patent risks". BioPharmaDive. Archived from the original on 2018-12-01. Retrieved 2021-04-15.
- ↑ Helfand C. "Copaxone". FiercePharma. Archived from the original on 2020-08-09. Retrieved 2021-04-15.
- ↑ Decker S (1 September 2016). "Teva loses decision on validity of 302 copaxone patent". Bloomberg Markets. Archived from the original on 2019-07-09. Retrieved 2021-04-15.
- ↑ Decker S, Flanagan C, Benmeleh Y (30 January 2017). "Teva loses ruling invalidating patents on copaxone drug". Bloomberg Markets. Archived from the original on 2021-09-23. Retrieved 2021-04-15.
- ↑ "Teva loses patent ruling". Briefcase. The Philadelphia Inquirer. Bloomberg News. September 2, 2017. p. A12. Archived from the original on June 24, 2018. Retrieved June 23, 2018 – via Newspapers.com (Publisher Extra).
- ↑ "U.S. appeals court upholds ruling that canceled Teva Copaxone patents". Reuters. October 12, 2018. Archived from the original on October 12, 2018. Retrieved October 12, 2018.
- ↑ "In Re: Copaxone Consolidated Cases" (PDF). United States Court of Appeals for the Federal Circuit. October 12, 2018. Archived (PDF) from the original on December 22, 2018. Retrieved October 12, 2018.
External links
| Identifiers: |
|
|---|
- "Glatiramer acetate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-03-24. Retrieved 2021-04-15.
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed DrugBank identifier
- Chemicals that do not have a ChemSpider ID assigned
- Articles with changed EBI identifier
- Articles with specifically marked weasel-worded phrases from October 2020
- Articles with invalid date parameter in template
- Immunostimulants
- Drugs with unknown mechanisms of action
- Israeli inventions
- RTT
- World Health Organization essential medicines
![Nicolau syndrome associated subcutaneous glatiramer acetate injection[15] a) Nicolau Syndrome on left abdomen b) erythematous, purpuric and haemorrhagic patch, at site of subcutaneous glatiramer acetate c) 3 weeks after GA injection livedoid patch disappeared](https://nccommons.org/media/thumb/7/7a/PMC4668705_12883_2015_504_Fig1_HTML.png/250px-PMC4668705_12883_2015_504_Fig1_HTML.png)
