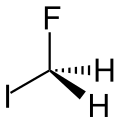
Fluoroiodomethane
Jump to navigation
Jump to search
| |
| Names | |
|---|---|
| Preferred IUPAC name
Fluoro(iodo)methane | |
| Other names
Fluoroiodomethane
Fluoro-iodo-methane Fluoromethyl iodide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.201.539 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH2FI | |
| Molar mass | 159.93 g/mol |
| Boiling point | 53.4 °C (128.1 °F; 326.5 K) |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H301, H311, H330 | |
| P260, P264, P270, P271, P280, P284, P301+P310, P302+P352, P304+P340, P310, P312, P320, P321, P322, P330, P361, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Fluoroiodomethane is the halomethane with the formula FCH2I. Also classified as a fluoroiodocarbon (FIC), it is a colorless liquid. It is a reagent for the introduction of the fluoromethyl (FCH2) group.
Synthesis and uses
It is prepared by fluorination of methylene iodide.[1]
Its isotopomer [18F]fluoroiodomethane is used for fluoromethylation of radiopharmaceuticals.
Additional reading
- Zheng L.; Berridge M. S. (January 2000). "Synthesis of [18F]fluoromethyl iodide, a synthetic precursor for fluoromethylation of radiopharmaceuticals". Applied Radiation and Isotopes. 52 (1): 55–61(7). doi:10.1016/S0969-8043(99)00061-5. PMID 10670923.
- Chin F. T.; Morse Ch. L.; Shetty H. U.; Pike V. W. (December 2005). "Automated radiosynthesis of [18F]SPA-RQ for imaging human brain NK1 receptors with PET". Journal of Labelled Compounds and Radiopharmaceuticals. 49 (1): 17–31(15). doi:10.1002/jlcr.1016. Retrieved 2007-06-29.[dead link]
- Tedder, J. M.; Sloan, J. P.; Walton, J. C. (1975). "Free Radical Addition to Olefins, Part XVII. Addition of Fluoroiodomethane to Fluoroethylenes". Journal of the Chemical Society: 1846–1850.
References
- ^ Landelle, Gregory; Paquin, Jean-Francois (2011). "Fluoroiodomethane". Encyclopedia of Reagents for Organic Synthesis. e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn01273. ISBN 978-0471936237.
Categories:
- Articles without EBI source
- Articles without KEGG source
- Articles without UNII source
- ECHA InfoCard ID from Wikidata
- Chembox having GHS data
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Short description matches Wikidata
- All articles with dead external links
- Articles with dead external links from February 2019
- Halomethanes
- Organofluorides
- Organoiodides
- All stub articles
- Organohalide stubs