Epidermolytic hyperkeratosis
| Epidermolytic Ichthyosis (EI) | |
|---|---|
| Other names: Bullous epidermis ichthyosis, bullous epidermis ichthyosis (BEI), epidermolytic hyperkeratosis (EHK), bullous congenital ichthyosiform erythroderma (BCIE),[1] bullous ichthyosiform erythroderma[2]: 482 bullous congenital ichthyosiform erythroderma Brocq[3] | |
 | |
| Specialty | Dermatology |
Epidermolytic ichthyosis (EI) is a rare and severe form of ichthyosis this skin disease affects around 1 in 300,000 people.
The condition is caused by a genetic mutation. The condition involves the clumping of keratin filaments.[4][5]: 562
While some research has been done into possible gene therapy treatments, the work hasn't yet been successfully developed to the stage where it can be routinely given to patients.
Signs and symptoms
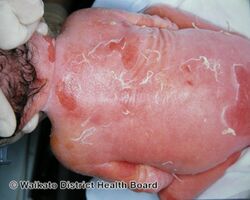
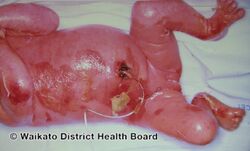
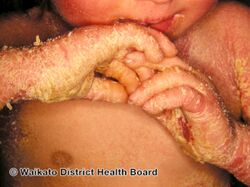
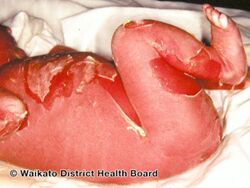
Epidermolytic hyperkeratosis is a skin disorder that is present at birth. Affected babies may have very red skin (erythroderma) and severe blisters. Because newborns with this disorder are missing the protection provided by normal skin, they are at risk of becoming dehydrated and developing infections in the skin or throughout the body (sepsis).
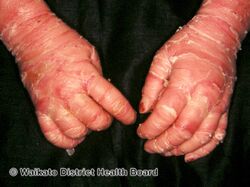
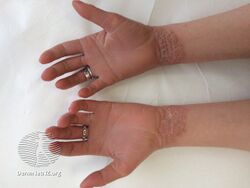
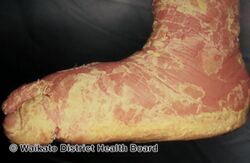
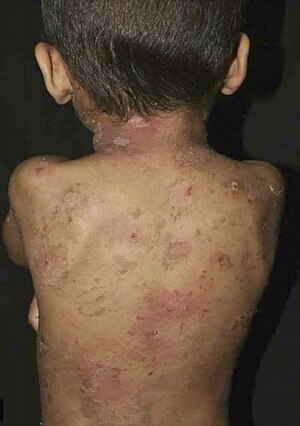
As affected individuals get older, blistering is less frequent, erythroderma becomes less evident, and the skin becomes thick (hyperkeratotic), especially over joints, on areas of skin that come into contact with each other, or on the scalp or neck. This thickened skin is usually darker than normal. Bacteria can grow in the thick skin, often causing a distinct odor.
Epidermolytic hyperkeratosis can be categorized into two types :-
- People with PS-type epidermolytic hyperkeratosis have thick skin on the palms of their hands and soles of their feet (palmoplantar or palm/sole hyperkeratosis) in addition to other areas of the body.
- People with the other type, NPS-type, do not have extensive palmoplantar hyperkeratosis, but do have hyperkeratosis on other areas of the body.
Epidermolytic hyperkeratosis is part of a group of conditions called ichthyoses, which refers to the scaly skin seen in individuals with related disorders. However, in epidermolytic hyperkeratosis, the skin is thick but not scaly as in some of the other conditions in the group. http://ghr.nlm.nih.gov/condition/epidermolytic-hyperkeratosis Archived 2020-08-10 at the Wayback Machine [6]
-
Epidermolytic hyperkeratosis
-
Epidermolytic hyperkeratosis
-
Epidermolytic hyperkeratosis
-
Epidermolytic hyperkeratosis
-
Epidermolytic hyperkeratosis
-
Epidermolytic hyperkeratosis
-
Epidermolytic hyperkeratosis
Genetics
It is possible to classify epidermolytic hyperkeratosis based upon palm and sole hyperkeratosis.[7]
This is a dominant[8] genetic condition caused by mutations in the genes encoding the proteins keratin 1 or keratin 10.
- Keratin 1 is associated with the variants affecting the palms and soles.
- Keratin 10 is associated with the variants in which these are unaffected.
Diagnosis
The condition can be diagnosed via exam that reveals; generalized redness; thick, generally dark, scales that tend to form parallel rows of spines or ridges, especially near large joints; the skin is fragile and blisters easily following trauma; extent of blistering and amount of scale is variable.[citation needed]
Treatment
Oral retinoids have proven effective in treating this disorder. Depending on the side effects they may improve the quality of life.[9] Examples are etretinate, acitretin, isotretinoin
Maintenance
Until gene therapy solutions become reality, people with EHK must treat their fragile skin carefully. Most have learned that taking regular extended baths allows patients to care for their fragile skin and keep it manageable. Baths that include sea salt seem to improve the process of softening and removing the thickened skin. Ointments like Petroleum Jelly, Aveeno, and other barrier type ointment help hold the moisture in the skin after a bath.
Research
Gene therapy is being studied for EHK.
Over the past 10 years since the first EHK mouse model was developed, many ideas have been discussed about how best to cure EHK. Back as far as 1994 researchers were discussing new promising ideas such as topical lotions that would deliver ribozymes in a liposom cream. Ribozymes are a small piece of synthetic RNA which can digest RNA molecules. When cells make a protein from a gene on a chromosome sitting in the nucleus, the gene is first transcribed as a piece of RNA. This RNA is then translated into a protein. Ribozymes can be designed to destroy RNA molecules with specific sequences. In theory, this will stop the production of the protein encoded by the mutant alleles of the gene.
Successful gene therapy solutions have been recently achieved on mouse models by Jiang Chen M.D., a post-doctoral fellow in the laboratory of Dennis Roop, Ph.D., in the Center for Cutaneous Molecular Biology at Baylor College of Medicine. In 1998 they developed an inducible mouse model for epidermolysis hyperkeratosis which is viable, because the expression of a mutant K10 allele can be restricted to a focal area of the skin. "Once the mutant K10 allele is activated in epidermal stem cells by topical application of an inducer, these stem cells continuously give rise to defective progeny that form hyperkeratotic lesions which persist for the life of the mouse. It was observed that partial suppression of the mutant K10 gene may be sufficient to eliminate the disorder."
To test this observation, Dr. Chen and his team of researchers developed siRNAs that target the mutant K10 gene products for degradation, without affecting normal K10 gene products. Dr. Chen observed that under these conditions, an efficient knock-down of mutant, but not normal, K10 genes could be achieved. The results allowed the normal K10 genes to function properly building healthy skin tissues. He claims that these results may prove to be a very vital step forward in forging a novel gene therapy and possible permanent corrective therapy for this debilitating skin disorder.
The challenge has always been how to deliver the siRNA using a topical method or retroviral vectors and ex vivo gene transfer.[10] In 2011/12 a team at Northwestern University claim to have solved the topical delivery of siRNA dilemma. Personalized siRNA can be delivered in a commercial moisturizer or phosphate-buffered saline, and do not require barrier disruption or transfection agents, such as liposomes, peptides, or viruses. "Topical application of nucleic acids offers many potential therapeutic advantages for suppressing genes in the skin, and potentially for systemic gene delivery. However, the epidermal barrier typically precludes entry of gene-suppressing therapy unless the barrier is disrupted. We now show that spherical nucleic acid nanoparticle conjugates (SNA-NCs), gold cores surrounded by a dense shell of highly oriented, covalently immobilized siRNA, freely penetrate almost 100% of keratinocytes in vitro, mouse skin, and human epidermis within hours after application."[11] This new discovery may soon offer hope to all with mono-genetic diseases such as EHK. This may lead to promising personalized, topically delivered gene therapy of cutaneous tumors, skin inflammation, and dominant negative genetic skin disorders.[12]
See also
- Hyperkeratosis
- Ichthyosis
- Ichthyosis bullosa of Siemens
- Isotretinoin (Accutane)
- List of cutaneous conditions
- List of cutaneous conditions caused by mutations in keratins
- List of verrucous carcinoma subtypes
- Nonbullous ichthyosiform erythroderma
References
- ↑ Rapini, Ronald P.; Bolognia, Jean L.; Jorizzo, Joseph L. (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. ISBN 978-1-4160-2999-1.
- ↑ Freedberg, et al. (2003). Fitzpatrick's Dermatology in General Medicine. (6th ed.). McGraw-Hill. ISBN 0-07-138076-0.
- ↑ synd/1036 at Who Named It?
- ↑ Cheng J, Syder AJ, Yu QC, Letai A, Paller AS, Fuchs E (September 1992). "The genetic basis of epidermolytic ichthyosis: a disorder of differentiation-specific epidermal keratin genes". Cell. 70 (5): 811–9. doi:10.1016/0092-8674(92)90314-3. PMID 1381287. S2CID 31906051.
- ↑ James, William; Berger, Timothy; Elston, Dirk (2005). Andrews' Diseases of the Skin: Clinical Dermatology. (10th ed.). Saunders. ISBN 0-7216-2921-0.
- ↑ "What is epidermolytic hyperkeratosis?". Archived from the original on 2020-08-10. Retrieved 2011-11-03.
- ↑ DiGiovanna JJ, Bale SJ (August 1994). "Clinical heterogeneity in epidermolytic hyperkeratosis". Arch Dermatol. 130 (8): 1026–35. doi:10.1001/archderm.130.8.1026. PMID 8053700.
- ↑ Ross R, DiGiovanna JJ, Capaldi L, Argenyi Z, Fleckman P, Robinson-Bostom L (July 2008). "Histopathologic characterization of epidermolytic hyperkeratosis: a systematic review of histology from the National Registry for Ichthyosis and Related Skin Disorders". J. Am. Acad. Dermatol. 59 (1): 86–90. doi:10.1016/j.jaad.2008.02.031. PMC 2517215. PMID 18571597.
- ↑ Brecher AR, Orlow SJ (2003). "Oral retinoid therapy for dermatologic conditions in children and adolescents". J. Am. Acad. Dermatol. 49 (2): 171–82, quiz 183–6. doi:10.1067/S0190-9622(03)01564-0. PMID 12894062.
- ↑ Hengge, U. R. (2006). "Gene therapy progress and prospects: the skin - easily accessible, but still far away". Gene Therapy. 13 (22): 1555–1563. doi:10.1038/sj.gt.3302855. PMID 16957767.
- ↑ Zheng, Dan; Giljohann, David A.; Chen, David L.; Massich, Matthew D.; Wang, Xiao-Qi; Iordanov, Hristo; Mirkin, Chad A.; Paller, Amy S. (2012). "Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation". Proceedings of the National Academy of Sciences. 109 (30): 11975–11980. Bibcode:2012PNAS..10911975Z. doi:10.1073/pnas.1118425109. PMC 3409786. PMID 22773805.
- ↑ "FIRST Funded Research | Foundation for Ichthyosis & Related Skin Types (FIRST)". Archived from the original on 2020-10-30. Retrieved 2021-05-31.
External links
| Classification | |
|---|---|
| External resources |