Distributive shock
| Distributive shock | |
|---|---|
| Other names: High space shock, warm shock, vasodilatory shock[1] | |
| Video explanation of shock | |
| Symptoms | Confusion, fast heart rate, warm arms and legs, altered body temperature, low blood pressure[2] |
| Complications | Multiple organ dysfunction syndrome[2] |
| Causes | Sepsis, anaphylaxis, spinal injury, toxic shock syndrome, adrenal insufficiency, pancreatitis, overdoses of certain medications[1][2] |
| Diagnostic method | Normal or above normal output from the heart[3] |
| Differential diagnosis | Other types of shock[2] |
| Treatment | Intravenous fluids, norepinephrine, epinephrine, antibiotics, hydrocortisone[2] |
Distributive shock is a type of shock in which small blood vessels do not regulate blood flow appropriately.[1] This results in not enough blood flow to body tissues.[2] Symptoms may include confusion, fast heart rate, warm arms and legs, altered body temperature, and low blood pressure.[2] Complications may include multiple organ dysfunction syndrome.[2]
The most common cause sepsis and anaphylaxis.[2] Other causes include spinal injury, known as neurogenic shock, toxic shock syndrome, adrenal insufficiency, pancreatitis, and overdoses of certain medications such as calcium channel blockers.[1][2] Distributive shock differs from the other three categories of shock in that there is normal or above normal output from the heart.[3]
Treatment often begins with intravenous fluids.[2] Other measure may include norepinephrine or epinephrine.[2] Other treatments depend on the underlying cause such as antibiotics or hydrocortisone.[2] Distributive shock makes up about 66% of cases of shock in the intensive care unit.[4] Death can occur in 20% to 80% of cases, depending on the cause.[2]
Signs and symptoms
Symptoms may include confusion, fast heart rate, warm arms and legs, altered body temperature, and low blood pressure.[2] Complications may include multiple organ dysfunction syndrome.[2]
Causes
In addition to sepsis, distributive shock can be caused by systemic inflammatory response syndrome (SIRS) due to conditions other than infection such as pancreatitis, burns or trauma.[5] Other causes include, toxic shock syndrome (TSS), anaphylaxis (a sudden, severe allergic reaction), adrenal insufficiency, reactions to drugs or toxins, heavy metal poisoning, hepatic (liver) insufficiency and damage to the central nervous system.[5] Causes of adrenal insufficiency leading to distributive shock include acute worsening of chronic adrenal insufficiency, destruction or removal of the adrenal glands, suppression of adrenal gland function due to exogenous steroids, hypopituitarism and metabolic failure of hormone production.[5]
Pathophysiology
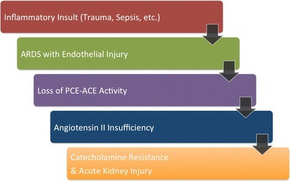
The cause of inadequate tissue perfusion (blood delivery to tissues) in distributive shock is a lack of normal responsiveness of blood vessels to vasoconstrictive agents and direct vasodilation.[7]
There are four types of distributive shock. The most common, septic shock, is caused by an infection, most frequently by bacteria, but viruses, fungi and parasites have been implicated.[5] Infection sites most likely to lead to septic shock are chest, abdomen and genitourinary tract.[5] In septic shock the blood flow in the microvasculature is abnormal with some capillaries underperfused and others with normal to high blood flow.[8] The endothelial cells lining the blood vessels become less responsive to vasocontrictive agents, lose their glycocalyx (normal coating) and negative ionic charge, become leaky and cause extensive over-expression of nitric oxide.[3] The coagulation cascade is also disrupted.[7] Tissue factor that initiates the clotting cascade is produced by activated monocytes and the endothelial cells lining the blood vessels while antithrombin and fibrinolysis are impaired.[7] Disseminated intravascular coagulation (DIC) can result from the thrombin produced in the inflammatory response.[7] The ability of red blood cells to change shape decreases and their tendency to clump together increases, inhibiting their flow through the microvasculature.[8]
In anaphylactic shock low blood pressure is related to decreased systemic vascular resistance (SVR) triggered primarily by a massive release of histamine by mast cells activated by antigen-bound immunoglobulin E and also by increased production and release of prostaglandins.[7]
Neurogenic shock is caused by the loss of vascular tone normally supported by the sympathetic nervous system due to injury to the central nervous system especially spinal cord injury.[7][9] Rupture of a hollow organ, with subsequent evacuation of contents in the peritoneal cavity could also determine neurogenic shock, a subtype of distributive shock. This happens due to the widespread peritoneal irritation by the ruptured viscus contents, as in peptic ulcer perforation, with consequent strong vagal activation, and generalized, extensive peripheral vasodilation and bradycardia.[10][11] Thus, in neurogenic shock, there is decreased systemic vascular resistance, CVP is typically decreased, CO decreased or normal, and PAOP decreased.[3]
Distributive shock associated with adrenal crisis results from inadequate steroid hormones.
Diagnosis
Types
Elbers and Ince have identified five classes of abnormal microcirculatory flow in distributive shock using side stream dark field microscopy.
- Class I: all capillaries are stagnant when there is normal or sluggish venular flow.
- Class II: there are empty capillaries next to capillaries that have flowing red blood cells.
- Class III: there are stagnant capillaries next to capillaries with normal blood flow.
- Class IV: hyperdynamic flow in capillaries adjacent to capillaries that are stagnant.
- Class V: widespread hyperdynamic flow in the microcirculatory system.[3]
According to the cause, there are 4 types of distributive shock:
- Neurogenic shock: Decreased sympathetic stimulation leading to decreased vasal tone.
- Anaphylactic shock
- Septic shock
- Shock due to adrenal crisis
Treatment
The main goals of treatment in distributive shock are to reverse the underlying cause and achieve hemodynamic stabilization.[12] Immediate treatment involves fluid resuscitation and the use of vasoactive drugs, both vasopressors and inotropes.[13] Hydrocortisone is used for people whose hypotension does not respond to fluid resuscitation and vasopressors.[14] Opening and keeping open the microcirculation is a consideration in the treatment of distributive shock, as a result limiting the use of vasopressors has been suggested.[3] Control of inflammation, vascular function and coagulation to correct pathological differences in blood flow and microvascular shunting has been pointed to as a potentially important adjunct goal in the treatment of distributive shock.[3]
People with septic shock are treated with antimicrobial drugs to treat the causative infection.[15] Some sources of infection require surgical intervention including necrotizing fasciitis, cholangitis, abscess, intestinal ischemia, or infected medical devices.[16]
Anaphylactic shock is treated with epinephrine.[17] The use of vasopressin together with norepinephrine rather than norepinephrine alone appears to decrease the risk of atrial fibrillation but with few other benefits.[18]
Prognosis
Septic shock is associated with significant mortality and is the leading non cardiac cause of death in intensive care units (ICUs).[19]
Research
The choice of fluids for resuscitation remains an area of research, the Surviving Sepsis Campaign an international consortium of experts, did not find adequate evidence to support the superiority crystalloid fluids versus colloid fluids.[13] Drugs such as, pyridoxalated hemoglobin polyoxyethylene, which scavenge nitric oxide from the blood have been investigated.[20] As well as methylene blue which may inhibit the nitric oxide-cyclic guanosine monophosphate (NO-cGMP) pathway which has been suggested to play a significant role in distributive shock.[21]
References
- ↑ 1.0 1.1 1.2 1.3 International Trauma Life Support for Emergency Care Providers (8 ed.). Pearson Education Limited. 2018. pp. 175, 177. ISBN 978-1292-17084-8.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 Smith, N; Lopez, RA; Silberman, M (January 2020). "Distributive Shock". PMID 29261964.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Elbers, Paul W.G.; Ince, Can (19 July 2006). "Mechanisms of critical illness--classifying microcirculatory flow abnormalities in distributive shock". Critical Care. 10 (4): 221. doi:10.1186/cc4969. PMC 1750971. PMID 16879732.
- ↑ McEvoy, Matthew D.; Furse, Cory M. (2017). Advanced Perioperative Crisis Management. Oxford University Press. p. 67. ISBN 978-0-19-022645-9. Archived from the original on 2021-08-28. Retrieved 2021-01-05.
- ↑ 5.0 5.1 5.2 5.3 5.4 Kanaparthi et al. 2013, Overview: Etiology.
- ↑ Chawla, Lakhmir S.; Busse, Laurence W.; Brasha-Mitchell, Ermira; Alotaibi, Ziyad (27 May 2016). "The use of angiotensin II in distributive shock". Critical Care. 20 (1). doi:10.1186/s13054-016-1306-5. ISSN 1364-8535. Retrieved 28 October 2022.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Kanaparthi et al. 2013, Overview: Pathophysiology.
- ↑ 8.0 8.1 Ince, Can (25 August 2005). "The microcirculation is the motor of sepsis". Critical Care. 4 (Supp 4): S13-9. doi:10.1186/cc3753. PMC 3226164. PMID 16168069.
- ↑ Weaver, Lynne C.; Fleming, Jennifer C.; Mathias, Christopher J.; Krassioukov, Andrie V. (2012). "Ch. 13: Disordered Cardiovascular Control After Spinal Cord Injury". In Verhaagen, Joost; McDonald, John W. (eds.). Spinal Cord Injury. Handbook of Clinical Neurology. Vol. 109. pp. 213–33. doi:10.1016/B978-0-444-52137-8.00013-9. ISBN 9780444521378. PMID 23098715.
- ↑ De-Giorgio F, Lodise M, Pascali VL, Spagnolo AG, d'Aloja E, Arena V (2015). "An Unusual Case Showing Fatal Rupture of a Gastric Ulcer or Gastromalacia? The Importance/Role of Histology for Differential Diagnosis". J Forensic Sci. 60 (1): 240–2. doi:10.1111/1556-4029.12665. PMID 25388056.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Civetta, Taylor, & Kirby's Critical Care, 4th Edition. Chapter 59 Neurogenic Shock. Lippincott Williams & Wilkins 2009
- ↑ Kanaparthi et al. 2013, Treatment: Approach Considerations.
- ↑ 13.0 13.1 Kanaparthi et al. 2013, Treatment: Resuscitation.
- ↑ Kanaparthi et al. 2013, Treatment: Corticosteroids.
- ↑ Kanaparthi et al. 2013, Treatment: Antimicrobial Treatment.
- ↑ Kanaparthi et al. 2013, Treatment: Surgical Control of Shock Sources.
- ↑ Kanaparthi et al. 2013, Treatment: Treatment of Anaphylaxis.
- ↑ McIntyre, WF; Um, KJ; Alhazzani, W; Lengyel, AP; Hajjar, L; Gordon, AC; Lamontagne, F; Healey, JS; Whitlock, RP; Belley-Côté, EP (8 May 2018). "Association of Vasopressin Plus Catecholamine Vasopressors vs Catecholamines Alone With Atrial Fibrillation in Patients With Distributive Shock: A Systematic Review and Meta-analysis". JAMA. 319 (18): 1889–1900. doi:10.1001/jama.2018.4528. PMC 6583502. PMID 29801010.
- ↑ Kanaparthi, Lalit K.; Klaus-Dieter, Lessnau; Peralta, Ruben (12 February 2013), Pinsky, Michael R. (ed.), "Distributive Shock: Overview: Background", Medscape Reference, Medscape, archived from the original on 2021-08-28, retrieved 2014-04-28.
- ↑ Kinasewitz, Gary T.; Privalle, Christopher T.; Imm, Amy; Steingrub, Jay S.; Malcynski, John T.; Balk, Robert A.; DeAngelo, Joseph (July 2008). "Multicenter, randomized, placebo-controlled study of the nitric oxide scavenger pyridoxalated hemoglobin polyoxyethylene in distributive shock". Critical Care Medicine. 36 (7): 1999–2007. doi:10.1097/CCM.0b013e31817bfe84. PMID 18552688.
{{cite journal}}: Unknown parameter|displayauthors=ignored (help) - ↑ Jang, D.H.; Nelson, L.S.; Hoffman, R.S. (September 2013). "Methylene blue for distributive shock: A potential new use of an old antidote". Journal of Medical Toxicology. 9 (3): 242–9. doi:10.1007/s13181-013-0298-7. PMC 3770994. PMID 23580172.
External links
- Surviving Sepsis Campaign Archived 2021-02-27 at the Wayback Machine