Phenytoin
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /fəˈnɪtoʊɪn, ˈfɛnɪtɔɪn/ |
| Trade names | Originally Dilantin, many names worldwide[1] |
| |
| Clinical data | |
| Drug class | Anticonvulsant[2] |
| Main uses | Seizures including status epilepticus[3] |
| Side effects | Nausea, stomach pain, loss of appetite, poor coordination, increased hair growth, enlargement of the gums[2] |
| Pregnancy category |
|
| Routes of use | By mouth, intravenous |
| Onset of action | 10 to 30 min (IV)[4] |
| Duration of action | 24 hr[4] |
| Defined daily dose | 300 mg[5] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682022 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 70–100% oral, 24.4% for rectal administration |
| Protein binding | 95%[2] |
| Metabolism | Liver |
| Elimination half-life | 10–22 hours[2] |
| Excretion | Primarily through the bile, urinary |
| Chemical and physical data | |
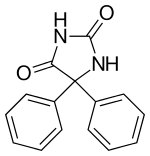
| Formula | C15H12N2O2 |
| Molar mass | 252.273 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Phenytoin (PHT), sold under the brand name Dilantin among others,[1] is an anti-seizure medication.[2] It is useful for the prevention of tonic-clonic seizures and focal seizures, but not absence seizures.[2] The intravenous form, fosphenytoin, is used for status epilepticus that does not improve with benzodiazepines.[2] It may also be used for certain heart arrhythmias or neuropathic pain.[2] It can be taken intravenously or by mouth.[2] The intravenous form generally begins working within 30 minutes and is effective for 24 hours.[4] Blood levels can be measured to determine the proper dose.[2]
Common side effects include nausea, stomach pain, loss of appetite, poor coordination, increased hair growth, and enlargement of the gums.[2] Potentially serious side effects include sleepiness, self harm, liver problems, bone marrow suppression, low blood pressure, and toxic epidermal necrolysis.[2] There is evidence that use during pregnancy results in abnormalities in the baby.[2] It appears to be safe to use when breastfeeding.[2] Alcohol may interfere with the medication's effects.[2]
Phenytoin was first made in 1908 by the German chemist Heinrich Biltz and found useful for seizures in 1936.[6][7] It is on the World Health Organization's List of Essential Medicines.[8] Phenytoin is available as a generic medication and usually not too expensive.[9] The wholesale cost in the developing world is between US$0.003 and US$0.15 per dose.[10] A month of treatment is about US$30 in the United States.[2] In 2017, it was the 221st most commonly prescribed medication in the United States, with more than two million prescriptions.[11][12]
Medical uses
Seizures
- Tonic-clonic seizures: Mainly used in the prophylactic management of tonic-clonic seizures with complex symptomatology (psychomotor seizures). A period of 5–10 days may be required to achieve anticonvulsant effects.
- Focal seizures: Mainly used to protect against the development of focal seizures with complex symptomatology (psychomotor and temporal lobe seizures). Also effective in controlling partial seizures with autonomic symptoms.
- Absence seizures: Not used in treatment of pure absence seizures due to risk for increasing frequency of seizures. However, can be used in combination with other anticonvulsants during combined absence and tonic-clonic seizures.
- Seizures during surgery: A 2018 meta-analysis found that early antiepileptic treatment with either phenytoin or phenobarbital reduced the risk of seizure in the first week after neurosurgery for brain tumors.[13]
- Status epilepticus: Considered after failed treatment using a benzodiazepine due to slow onset of action.[14]
Other
- Abnormal heart rhythms: may be used in the treatment of ventricular tachycardia and sudden episodes of atrial tachycardia after other antiarrhythmic medications or cardioversion has failed. It is a class 1b antiarrhythmic.[15]
- Digoxin toxicity: IV formulation is drug of choice for arrhythmias caused by cardiac glycoside toxicity.
- Trigeminal neuralgia: Second choice drug to carbamazepine.[16]
Dosage
For status epilepticus a loading dose of 15-20 mg/kg IV; if ineffective, can give 10 mg/kg IV 20 min after first dose.[17] For adults who weight 50 kg this is a dose of 1 gram.[3] Maintenance treatment is 100 mg PO/IV q 6-8 hrs starting 12 hours after loading dose.[17]
For a seizure disorder 300 to 400 mg/day divided into 2-3 doses/day.[17] In children may give 4-7 mg/kg/day divided into 2-3 doses/day.[17] Doses are than adjusted based on response and blood levels.[17] The maximum dose is listed as 600 mg.[18] There is an extended and immediate release formulation.[17]
Treatment levels may also be achieved rapidly by giving 400 mg by mouth, followed two hour later by 300 mg, and two hours after that 300 mg.[2] Than the usual maintenance is used.[2]
The defined daily dose is 300 mg by mouth or by injection.[5]
Side effects
Common side effects include nausea, stomach pain, loss of appetite, poor coordination, increased hair growth, and enlargement of the gums.[2] Potentially serious side effects include sleepiness, self harm, liver problems, bone marrow suppression, low blood pressure, and toxic epidermal necrolysis.[2] There is evidence that use during pregnancy results in abnormalities in the baby.[2] Its use appears to be safe during breastfeeding.[2] Alcohol may interfere with the medication's effects.[2]
Heart and blood vessels
Severe low blood pressure and abnormal heart rhythms can be seen with rapid infusion of IV phenytoin. IV infusion should not exceed 50 mg/min in adults or 1–3 mg/kg/min (or 50 mg/min, whichever is slower) in children. Heart monitoring should occur during and after IV infusion. Due to these risks, oral phenytoin should be used if possible.[19]
Neurological
At therapeutic doses, phenytoin may produce nystagmus on lateral gaze. At toxic doses, patients experience vertical nystagmus, double vision, sedation, slurred speech, cerebellar ataxia, and tremor.[20] If phenytoin is stopped abruptly, this may result in increased seizure frequency, including status epilepticus.[19][21]
Phenytoin may accumulate in the cerebral cortex over long periods of time which can cause atrophy of the cerebellum. The degree of atrophy is related to the duration of phenytoin treatment and is not related to dosage of the medication.[22]
Phenytoin is known to be a causal factor in the development of peripheral neuropathy.[23]
Blood
Folate is present in food in a polyglutamate form, which is then converted into monoglutamates by intestinal conjugase to be absorbed by the jejunum. Phenytoin acts by inhibiting this enzyme, thereby causing folate deficiency, and thus megaloblastic anemia.[24] Other side effects may include: agranulocytosis,[25] aplastic anemia,[26] decreased white blood cell count,[27] and a low platelet count.[28]
Pregnancy
Phenytoin is a known teratogen. The syndrome consists of craniofacial anomalies (broad nasal bridge, cleft lip and palate, smaller than normal head) and a mild form of mental retardation (average IQ=71).[29] This syndrome resembles the well-described fetal alcohol syndrome[30] and has also been called the "fetal hydantoin syndrome". Some recommend avoiding polytherapy and maintaining the minimal dose possible during pregnancy, but acknowledge that current data do not provide clear answers.[31] Data now being collected by the Epilepsy and Antiepileptic Drug Pregnancy Registry may one day answer this question definitively.
Cancer
There is no good evidence that phenytoin is a human carcinogen.[32][33]
Mouth
Phenytoin has been associated with drug-induced gingival enlargement (overgrowth of the gums), probably due to above-mentioned folate deficiency; indeed, evidence from a randomized controlled trial suggests that folic acid supplementation can prevent gingival enlargement in children who take phenytoin.[34] Plasma concentrations needed to induce gingival lesions have not been clearly defined. Effects consist of the following: bleeding upon probing, increased gingival exudate, pronounced gingival inflammatory response to plaque levels, associated in some instances with bone loss but without tooth detachment.
Skin

Hypertrichosis, Stevens–Johnson syndrome, purple glove syndrome, rash, exfoliative dermatitis, itching, excessive hairiness, and coarsening of facial features can be seen in those taking phenytoin.
Phenytoin therapy has been linked to the life-threatening skin reactions Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). These conditions are significantly more common in patients with a particular HLA-B allele, HLA-B*1502.[35] This allele occurs almost exclusively in patients with ancestry across broad areas of Asia, including South Asian Indians.
Phenytoin is primarily metabolized to its inactive form by the enzyme CYP2C9. Variations within the CYP2C9 gene that result in decreased enzymatic activity have been associated with increased phenytoin concentrations, as well as reports of drug toxicities due to these increased concentrations.[36] The U.S. Food and Drug Administration (FDA) notes on the phenytoin drug label that since strong evidence exists linking HLA-B*1502 with the risk of developing SJS or TEN in patients taking carbamazepine, consideration should be given to avoiding phenytoin as an alternative to carbamazepine in patients carrying this allele.[37]
Immune system
Phenytoin has been known to cause drug-induced lupus.[38]
Phenytoin is also associated with induction of reversible IgA deficiency.[39]
Psychological
Phenytoin may increase risk of suicidal thoughts or behavior. People on phenytoin should be monitored for any changes in mood, the development or worsening depression, and/or any thoughts or behavior of suicide.[21]
Bones
Chronic phenytoin use has been associated with decreased bone density and increased bone fractures. Phenytoin induces metabolizing enzymes in the liver. This leads to increased metabolism of vitamin D, thus decreased vitamin D levels. Vitamin D deficiency, as well as low calcium and phosphate in the blood cause decreased bone mineral density.[21]
Special considerations
- Monitoring plasma concentrations: Narrow therapeutic index. Anticonvulsant effect: 10–20 µg/mL; Antiarrhythmic effect: 10–20 µg/mL
- Avoid giving intramuscular formulation unless necessary due to skin cell death and local tissue destruction.
- Elderly: May show earlier signs of toxicity.
- Obese: Use ideal body weight for dosing calculations.
- Pregnancy category D due to risk of fetal hydantoin syndrome and fetal bleeding. However, optimal seizure control is very important during pregnancy so drug may be continued if benefits outweigh the risks. Due to decreased drug concentrations during pregnancy, dose of phenytoin may need to be increased if only option for seizure control.
- Breast feeding: The manufacturer does not recommend breast feeding because low concentrations of phenytoin are excreted in breast milk.[21]
- Liver disease: Do not use oral loading dose. Consider using decreased maintenance dose.
- Kidney disease: Do not use oral loading dose. Can begin with standard maintenance dose and adjust as needed.
- IV use is contraindicated in patients with sinus bradycardia, SA block, second- or third-degree AV block, Stokes-Adams syndrome, or hypersensitivity to phenytoin, other hydantoins or any ingredient in the respective formulation.
Interactions
Phenytoin is an inducer of the CYP3A4 and CYP2C9 families of the P450 enzyme responsible for the liver's degradation of various drugs.[40]
A 1981 study by the National Institutes of Health showed that antacids administered concomitantly with phenytoin "altered not only the extent of absorption but also appeared to alter the rate of absorption. Antacids administered in a peptic ulcer regimen may decrease the AUC of a single dose of phenytoin. Patients should be cautioned against concomitant use of antacids and phenytoin."[41]
Warfarin (Coumadin) and trimethoprim increase serum phenytoin levels and prolong the serum half-life of phenytoin by inhibiting its metabolism. Consider using other options if possible.[42]
Mechanism of action
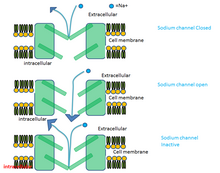
Phenytoin is believed to protect against seizures by causing voltage-dependent block of voltage gated sodium channels.[43] This blocks sustained high frequency repetitive firing of action potentials. This is accomplished by reducing the amplitude of sodium-dependent action potentials through enhancing steady state inactivation. Sodium channels exist in three main conformations: the resting state, the open state, and the inactive state.
Phenytoin binds preferentially to the inactive form of the sodium channel. Because it takes time for the bound drug to dissociate from the inactive channel, there is a time dependent block of the channel. Since the fraction of inactive channels is increased by membrane depolarization as well as by repetitive firing, the binding to the inactive state by phenytoin sodium can produce voltage-dependent, use-dependent and time-dependent block of sodium-dependent action potentials.[44]
The primary site of action appears to be the motor cortex where spread of seizure activity is inhibited.[45] Possibly by promoting sodium efflux from neurons, phenytoin tends to stabilize the threshold against hyperexcitability caused by excessive stimulation or environmental changes capable of reducing membrane sodium gradient. This includes the reduction of post-tetanic potentiation at synapses which prevents cortical seizure foci from detonating adjacent cortical areas. Phenytoin reduces the maximal activity of brain stem centers responsible for the tonic phase of generalized tonic-clonic seizures.[19]
Pharmacokinetics
Phenytoin elimination kinetics show mixed-order behaviour at therapeutic concentrations. A small increase in dose may lead to a large increase in drug concentration as elimination becomes saturated. The time to reach steady state is often longer than 2 weeks.[46][47][48][49]
History
Phenytoin (diphenylhydantoin) was first synthesized by German chemist Heinrich Biltz in 1908.[50] Biltz sold his discovery to Parke-Davis, which did not find an immediate use for it. In 1938, outside scientists including H. Houston Merritt and Tracy Putnam discovered phenytoin's usefulness for controlling seizures, without the sedative effects associated with phenobarbital.
According to Goodman and Gilman's Pharmacological Basis of Therapeutics
In contrast to the earlier accidental discovery of the antiseizure properties of potassium bromide and phenobarbital, phenytoin was the product of a search among nonsedative structural relatives of phenobarbital for agents capable of suppressing electroshock convulsions in laboratory animals.[51]
It was approved by the United States Food and Drug Administration in 1953 for use in seizures.
Jack Dreyfus, founder of the Dreyfus Fund, became a major proponent of phenytoin as a means to control nervousness and depression when he received a prescription for Dilantin in 1966. He has claimed to have supplied large amounts of the drug to Richard Nixon throughout the late 1960s and early 1970s, although this is disputed by former White House aides.[52] Dreyfus' experience with phenytoin is outlined in his book, A Remarkable Medicine Has Been Overlooked.[53] Despite more than $70 million in personal financing, his push to see phenytoin evaluated for alternative uses has had little lasting effect on the medical community. This was partially because Parke-Davis was reluctant to invest in a drug nearing the end of its patent life, and partially due to mixed results from various studies.
In 2008, the drug was put on the FDA's Potential Signals of Serious Risks List to be further evaluated for approval. The list identifies medications that the FDA has identified a potential safety issue, but does not mean that FDA has identified a causal relationship between the drug and the listed risk. To address this concern, the Warnings and Precautions section of the labeling for Dilantin injection was updated to include additional information about purple glove syndrome in November 2011.[54]
Society and culture
Cost
Phenytoin is available as a generic medication and usually not too expensive.[9] Wholesale it costs between US$0.003 and US$0.15 per dose.[10] A month of treatment is about US$30 in the United States.[2]
Since September 2012, the marketing licence in the UK has been held by Flynn Pharma Ltd, of Dublin, Ireland, and the product, although identical, has been called Phenytoin Sodium xxmg Flynn Hard Capsules. (The xxmg in the name refers to the strength—for example "Phenytoin sodium 25 mg Flynn Hard Capsules").[55] The capsules are still made by Pfizer's Goedecke subsidiary's plant in Freiburg, Germany and they still have Epanutin printed on them.[56] After Pfizer's sale of the UK marketing licence to Flynn Pharma, the price of a 28-pack of 25 mg phenytoin sodium capsules marked Epanutin rose from 66p (about $0.88) to £15.74 (about $25.06). Capsules of other strengths also went up in price by the same factor—2384%,[57] costing the UK's National Health Service an extra £43 million (about $68.44 million) a year.[58] The companies were referred to the Competition and Markets Authority who found that they had exploited their dominant position in the market to charge "excessive and unfair" prices.[59]
The Competition and Markets Authority (CMA) imposed a record £84.2 million fine on the manufacturer Pfizer, and a £5.2 million fine on the distributor Flynn Pharma and ordered the companies to reduce their prices.[60]
-
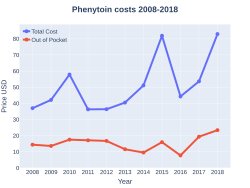
Phenytoin costs (US)
-
Phenytoin prescriptions (US)
Trade names
Phenytoin is marketed under many trade names worldwide.[1]
Research
Tentative evidence suggests that topical phenytoin is useful in wound healing in people with chronic skin wounds.[61][62] A meta-analysis also supported the use of phenytoin in managing various ulcers.[63]
Some clinical trials have explored whether phenytoin can be used as neuroprotector in multiple sclerosis.[64]
References
- ↑ 1.0 1.1 1.2 Drugs.com International trade names for phenytoin Archived 2016-02-23 at the Wayback Machine Page accessed Feb 27, 2016
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 "Phenytoin". The American Society of Health-System Pharmacists. Archived from the original on 2015-09-08. Retrieved Aug 22, 2015.
- ↑ 3.0 3.1 "PHENYTOIN injectable - Essential drugs". medicalguidelines.msf.org. Archived from the original on 28 August 2021. Retrieved 4 September 2020.
- ↑ 4.0 4.1 4.2 Marx, John A. (2010). Rosen's emergency medicine : concepts and clinical practice (7 ed.). Philadelphia: Mosby/Elsevier. p. 1352. ISBN 9780323054720. Archived from the original on 2020-08-19. Retrieved 2017-09-10.
- ↑ 5.0 5.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 6 November 2020. Retrieved 4 September 2020.
- ↑ Aicardi, Jean (2008). Epilepsy : a comprehensive textbook (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 1431. ISBN 9780781757775. Archived from the original on 2020-08-18. Retrieved 2017-09-10.
- ↑ Wolfson, Allan B. (2010). Harwood-Nuss' clinical practice of emergency medicine (5th ed.). Philadelphia, PA: Lippincott Williams & Wilkins. p. 1415. ISBN 9780781789431. Archived from the original on 13 November 2018. Retrieved 9 June 2016.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ 9.0 9.1 Hamilton, Richard J. (2013). Tarascon pocket pharmacopoeia (14 ed.). Burlington, MA.: Jones & Bartlett Learning. p. 294. ISBN 9781449673635. Archived from the original on 2018-11-13. Retrieved 2017-09-10.
- ↑ 10.0 10.1 "Phenytoin". International Drug Price Indicator Guide. Archived from the original on 26 February 2019. Retrieved 23 August 2015.
- ↑ "The Top 300 of 2020". ClinCalc. Archived from the original on 18 March 2020. Retrieved 11 April 2020.
- ↑ "Phenytoin - Drug Usage Statistics". ClinCalc. Archived from the original on 28 February 2020. Retrieved 11 April 2020.
- ↑ Joiner, EF; Youngerman, BE; Hudson, TS; Yang, J; Welch, MR; McKhann GM, 2nd; Neugut, AI; Bruce, JN (27 April 2018). "Effectiveness of perioperative antiepileptic drug prophylaxis for early and late seizures following oncologic neurosurgery: a meta-analysis". Journal of Neurosurgery. 130 (4): 1274–1282. doi:10.3171/2017.10.JNS172236. PMID 29701546.
- ↑ "Phenytoin". Lexi-Comp Online. Archived from the original on 4 March 2016. Retrieved 18 April 2014.
- ↑ McEvoy, GK (2004). "AHFS drug information 2004". American Society of Health-System Pharmacists: 2117–2120.
- ↑ Pharmacology and pharmacotheraputics 22ed edition pg:129 By R S Satoskar
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 "Phenytoin - WikEM". www.wikem.org. Archived from the original on 9 August 2020. Retrieved 5 August 2020.
- ↑ "PHENYTOIN oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 28 August 2021. Retrieved 23 August 2020.
- ↑ 19.0 19.1 19.2 "FDA drug label" (PDF). FDA. Archived (PDF) from the original on 19 April 2014. Retrieved 18 April 2014.
- ↑ "Dilantin Toxicity". Archived from the original on 2014-04-19.
- ↑ 21.0 21.1 21.2 21.3 Phenytoin [package insert]. New York, NY: Pfizer Inc.; 2013. Accessed November 2, 2014.
- ↑ De Marco, Felipe A; et al. (July 2003). "Cerebellar Volume and Long-Term Use of Phenytoin". European Journal of Epilepsy. 12 (5): 312–315. doi:10.1016/s1059-1311(02)00267-4. PMID 12810345. Retrieved 20 April 2014.
- ↑ Donofrio, Peter D. (2012) Textbook of Peripheral Neuropathy Archived 2017-09-08 at the Wayback Machine New York : Demos Medical Pub. page 208. ISBN 9781936287109
- ↑ Carl GF, Smith ML (1992). "Phenytoin-folate interactions: differing effects of the sodium salt and the free acid of phenytoin". Epilepsia. 33 (2): 372–375. doi:10.1111/j.1528-1157.1992.tb02330.x. PMID 1547769.
- ↑ Sharafuddin MJ, Spanheimer RG, McClune GL (1991). "Phenytoin-induced agranulocytosis: a nonimmunologic idiosyncratic reaction?". Acta Haematol. 86 (4): 212–3. doi:10.1159/000204838. PMID 1805490.
- ↑ Handoko KB, Souverein PC, van Staa TP, Meyboom RH, Leufkens HG, Egberts TC, van den Bemt PM (2006). "Risk of aplastic anemia in patients using antiepileptic drugs". Epilepsia. 47 (7): 1232–6. doi:10.1111/j.1528-1167.2006.00596.x. hdl:1874/27341. PMID 16886988.
- ↑ Workman, Linda M. and Lacharity, Linda A. (2015) Understanding Pharmacology: Essentials for Medication Safety Archived 2017-09-08 at the Wayback Machine St. Louis, Mo. : Elsevier. page 302. ISBN 9781455739769
- ↑ "Archived copy". Archived from the original on 2013-02-12. Retrieved 2013-07-08.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Beckmann CR, et al. (2002). Obstetrics and Gynecology (4th ed.). Baltimore: Lippincott Williams & Wilkins.
- ↑ CDC. (2004). Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis. Can be downloaded at "Archived copy". Archived from the original on 2007-05-05. Retrieved 2007-02-24.
{{cite web}}: CS1 maint: archived copy as title (link). - ↑ Adab N, Tudur SC, Vinten J, Williamson P, Winterbottom J (2004). Adab N (ed.). "Common Antiepileptic Drugs in Pregnancy in Women with Epilepsy". Cochrane Database of Systematic Reviews. 2004 (3): CD004848. doi:10.1002/14651858.CD004848. PMID 15266543.
- ↑ Report on Carcinogens, Eleventh Edition (PB2005-104914, 2004) p III-216.
- ↑ Maeda T, Sano N, Togei K, Shibata M, Izumi K, Otsuka H (1988). "Lack of carcinogenicity of phenytoin in (C57BL/6 x C3H)F1 mice". Journal of Toxicology and Environmental Health. 24 (1): 111–119. doi:10.1080/15287398809531144. PMID 3373561.
- ↑ Arya R, Gulati S, Kabra M, Sahu JK, Kalra V (2011). "Folic acid supplementation prevents phenytoin-induced gingival overgrowth in children". Neurology. 76 (15): 1338–1343. doi:10.1212/WNL.0b013e3182152844. PMC 3090066. PMID 21482950.
- ↑ Man CB, Kwan P, Baum L, et al. (2007). "Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese". Epilepsia. 48 (5): 1015–1018. doi:10.1111/j.1528-1167.2007.01022.x. PMID 17509004.
- ↑ Caudle, KE; Rettie, AE; Whirl-Carrillo, M; Smith, LH; Mintzer, S; Lee, MT; Klein, TE; Callaghan, JT (November 2014). "Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing". Clinical Pharmacology and Therapeutics. 96 (5): 542–8. doi:10.1038/clpt.2014.159. PMC 4206662. PMID 25099164.
- ↑ "DILANTIN- phenytoin sodium capsule, extended release". Archived from the original on 2 April 2015. Retrieved 12 March 2015.
- ↑ Scheinfeld N (2003). "Phenytoin in Cutaneous Medicine: Its Uses, Mechanisms and Side Effects". Dermatology Online Journal. 9 (3): 6. PMID 12952753. Archived from the original on 2008-08-29.
- ↑ Gilhus NE, Aarli JA (1981). "The reversibility of phenytoin-induced IgA deficiency". Journal of Neurology. 226 (1): 53–61. doi:10.1007/BF00313318. PMID 6181216.
- ↑ Cuttle, L; et al. (August 2000). "Phenytoin metabolism by human cytochrome P450: involvement of P450 3A and 2C forms in secondary metabolism and drug-protein adduct formation". Drug Metabolism and Disposition. 28 (8): 945–950. PMID 10901705.
- ↑ Carter, BL; et al. (1981). "Effect of antacids on phenytoin availability". Therapeutic Drug Monitoring. 3 (4): 333–340. doi:10.1097/00007691-198104000-00003. PMID 7336470.
- ↑ "Lexi-Comp Online Interaction Lookup". Lexi-Comp. Archived from the original on 2014-04-19.
- ↑ Rogawski MA, Löscher W (Jul 2004). "The neurobiology of antiepileptic drugs". Nat Rev Neurosci. 5 (7): 553–564. doi:10.1038/nrn1430. PMID 15208697.
- ↑ lippincots modern pharmacology with clinical applications pg no:377 5th Edition
- ↑ Dilantin. (2015). In MIMS. Retrieved from https://www.mims.com/Hongkong/drug/info/Dilantin/?type=full#Actions Archived 2014-08-10 at the Wayback Machine
- ↑ "Archive copy". Archived from the original on 2020-09-25. Retrieved 2013-01-17.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Chapter 67 Antiepileptic drug pharmacokinetics and therapeutic drug monitoring pp. 358–366 By Philip N. Patsalos View chapter as PDF Antiepileptic drug pharmacokinetics and therapeutic drug monitoring By Philip N. Patsalos
- ↑ "Archived copy". Archived from the original on 2013-03-01. Retrieved 2013-01-17.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ DONAHUE, S; FLOCKHART, D; ABERNETHY, D (December 1999). "Ticlopidine inhibits phenytoin clearance". Clinical Pharmacology & Therapeutics. 66 (6): 563–568. doi:10.1053/cp.1999.v66.103277001. PMID 10613611.
- ↑ Biltz H (1908). "Über die Konstitution der Einwirkungsprodukte von substituierten Harnstoffen auf Benzil und über einige neue Methoden zur Darstellung der 5,5-Diphenyl-hydantoine" [Constitution of the Products of the Interaction of Substituted Carbamides on Benzil and Certain New Methods for the Preparation of 5,5-Diphenylhydantoin]. Chemische Berichte (in German). 41 (1): 1379–1393. doi:10.1002/cber.190804101255. Archived from the original on 2020-08-06. Retrieved 2019-06-27.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ Goodman and Gilman's Pharmacological Basis of Therapeutics (10th ed.). New York: McGraw-Hill. 2001.
- ↑ Stout, David (2000-08-31). "2 Nixon Aides Skeptical About Report That He Took Drug". New York Times. Archived from the original on 2020-09-11. Retrieved 2020-02-06.
- ↑ Dreyfus J (1998). A Remarkable Medicine Has Been Overlooked: Including an Autobiography and the Clinical Section of the Broad Range of Use of Phenytoin. Continuum International Publishing Group. ISBN 978-0-8264-1069-6.
- ↑ "AERS data". FDA. Archived from the original on 19 April 2014. Retrieved 18 April 2014.
- ↑ Phenytoin Sodium Flynn Hard Capsules...Marketing authorisation holder, Flynn Pharma patient information leaflet at the electronic Medicines Compendium, 24 April 2014 Archived 15 January 2017 at the Wayback Machine. Accessed 13 May 2014.
- ↑ Healthcare Professionals Advised That Epanutin Capsules Are Only To Be Available Under The Generic Name, MediGuard, Durham, NC, 2 October 2012 Archived 14 May 2014 at the Wayback Machine.Accessed 13 May 2014.
- ↑ "Pharma firm hikes cost of epilepsy drug 24 times", Stephen Adams, Medical Correspondent, Daily Telegraph, London, 12 October 2012 Archived 9 June 2014 at the Wayback Machine. Accessed 13 May 2014.
- ↑ "Price of anti-epilepsy drug rockets", Mark Gould, Pulse, London, 21 November 2012 Archived 28 November 2012 at the Wayback Machine. Accessed 13 May 2014.
- ↑ White, Michael (12 August 2015). "Pharma market abuse isn't picked up fast enough". Health Service Journal. Archived from the original on 7 October 2015. Retrieved 6 October 2015.
- ↑ "CMA fines Pfizer and Flynn £90 million for drug price hike to NHS" (Press release). www.gov.uk. Archived from the original on 2016-12-07. Retrieved 2016-12-07.
- ↑ Shaw, J; Hughes, CM; Lagan, KM; Bell, PM (Nov 2007). "The clinical effect of topical phenytoin on wound healing: a systematic review". The British Journal of Dermatology. 157 (5): 997–1004. doi:10.1111/j.1365-2133.2007.08160.x. PMID 17854378.
- ↑ Bhatia, A; Prakash, S (Jul 15, 2004). "Topical phenytoin for wound healing". Dermatology Online Journal. 10 (1): 5. PMID 15347487.
- ↑ Thangaraju, Pugazhenthan; Tamilselvan, T; Venkatesan, S; Eswaran, T; singh, H; Giri, VC; showkath Ali, MK (Jul 16, 2015). "Topical Phenytoin for Managing Various Ulcers:A meta-analysis". Sudan Medical Monitor. 10 (2): 63–67. doi:10.4103/1858-5000.160951.
- ↑ Rhian Raftopoulos et al. Phenytoin for neuroprotection in patients with acute optic neuritis: a randomised, placebo-controlled, phase 2 trial, The Lancet Neurology, Volume 15, Issue 3, March 2016, Pages 259–269
Further reading
- Dean L (2016). "Phenytoin Therapy and HLA-B*15:02 and CYP2C9 Genotypes". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520374. Bookshelf ID: NBK385287. Archived from the original on 2020-10-26. Retrieved 2020-02-06.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- CS1 maint: archived copy as title
- CS1 maint: unrecognized language
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed EBI identifier
- Antiarrhythmic agents
- Anticonvulsants
- Aromatase inhibitors
- GABAA receptor positive allosteric modulators
- Hepatotoxins
- Hydantoins
- IARC Group 2B carcinogens
- Nephrotoxins
- Phenyl compounds
- Selective estrogen receptor modulators
- Sigma receptor ligands
- Sodium channel blockers
- Teratogens
- World Health Organization essential medicines
- RTT