Dexrazoxane
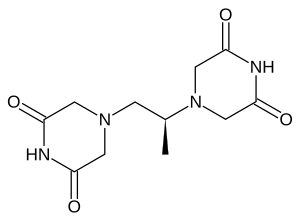 | |
| Names | |
|---|---|
| Trade names | Zinecard, Cardioxane, others |
| Other names | Dexrazoxane hydrochloride |
| |
| Clinical data | |
| Main uses | Prevent cardiomyopathy due to doxorubicin, extravasation of anthracyclines[1][2] |
| Side effects | Pain at site of injection[3] |
| Pregnancy category |
|
| Routes of use | Intravenous |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609010 |
| Legal | |
| License data |
|
| Legal status |
|
| Chemical and physical data | |
| Formula | C11H16N4O4 |
| Molar mass | 268.273 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dexrazoxane, sold under the brand name Zinecard among others, is a medication used to prevent cardiomyopathy due to doxorubicin and in extravasation of anthracyclines.[1][2] It is given by injection into a vein.[1]
Common side effects include pain at the site of injection, nausea, and diarrhea.[3][1] Other side effects may include bone marrow suppression.[3] In children in may increase the risk of further cancers.[4] It is an antidote to anthracyclines.[2]
Dexrazoxane was discovered in 1972.[4] It was approved for medical use in the United States in 1995 and Europe in 2006.[1][2] In the United States 500 mg costs about 420 USD as of 2021.[5] This amount in the United Kingdom is about £160.[6]
Medical uses
Dexrazoxane has been used to protect the heart against the cardiotoxic side effects of chemotherapeutic drugs such as anthracyclines,[7] such as daunorubicin or doxorubicin or other chemotherapeutic agents.[8] However, in July 2011 the European Medicines Agency (EMA) released a statement restricting use only in adult patients with cancer who have received > 300 mg/m2 doxorubicin or > 540 mg/m2 epirubicin and general approval for use for cardioprotection.[9][10] That showed a possibly higher rate of secondary malignancies and acute myelogenous leukemia in pediatric patients treated for different cancers with both dexrazoxane and other chemotherapeutic agents that are associated with secondary malignancies.[11] In 2017, based on evaluation of the data the European Commission issued an EU-wide decision to implement the recommendations of the Committee for Medicinal Products for Human Use (CHMP) on dexrazoxane and lifted its 2011-contraindication for primary prevention of anthracycline-induced cardiotoxicity with dexrazoxane in children and adolescents where high doses (≥ 300 mg/m3) of anthracyclines are anticipated.
Dexrazoxane was designated by the US FDA as an orphan drug for "prevention of cardiomyopathy for children and adults 0 through 16 years of age treated with anthracyclines".[12] This decision allows children to receive dexrazoxane starting with the first dose of anthracycline at the discretion of the treating provider. The label change by the agency announcing dexrazoxane as an approved cardio-oncology protectant has been followed by a review by the agency.[13] As of 2018 it is the only FDA and EMA approved cardioprotective treatment for anthracycline cardioprotection is dexrazoxane, which provides effective primary cardioprotection against anthracycline-induced cardiotoxicity without reducing anthracycline activity and without enhancing secondary malignancies.[14]
It is also used as a treatment of extravasation resulting from IV anthracycline chemotherapy.[15][16]
Dosage
For prevention of heart toxicity 10 mg/m2 of dexrazoxane is given for every 1 mg/m2 of doxorubicin that is planned to be used.[3]
Mechanism
As a derivative of EDTA, dexrazoxane chelates iron and thus reduces the number of metal ions complexed with anthracycline and, consequently, decrease the formation of superoxide radicals.[17] The exact chelation mechanism is unknown, but it has been postulated that dexrazoxane can be converted into ring-opened form intracellularly and interfere with iron-mediated free radical generation that is in part thought to be responsible for anthryacycline induced cardiomyopathy.[18] It was speculated that dexrazoxane could be used for further investigation to synthesize new antimalarial drugs.[19]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Dexrazoxane Monograph for Professionals". Drugs.com. Archived from the original on 8 May 2021. Retrieved 23 December 2021.
- ↑ 2.0 2.1 2.2 2.3 "Savene". Archived from the original on 12 November 2020. Retrieved 23 December 2021.
- ↑ 3.0 3.1 3.2 3.3 "DailyMed - DEXRAZOXANE- dexrazoxane for injection injection, powder, lyophilized, for solution". dailymed.nlm.nih.gov. Archived from the original on 26 March 2021. Retrieved 23 December 2021.
- ↑ 4.0 4.1 Kim, Kyu-Won; Roh, Jae Kyung; Wee, Hee-Jun; Kim, Chan (14 November 2016). Cancer Drug Discovery: Science and History. Springer. p. 250. ISBN 978-94-024-0844-7. Archived from the original on 11 January 2022. Retrieved 23 December 2021.
- ↑ "Dexrazoxane Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 22 April 2021. Retrieved 23 December 2021.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 984. ISBN 978-0857114105.
- ↑ Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. (July 2004). "The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia". The New England Journal of Medicine. 351 (2): 145–53. doi:10.1056/NEJMoa035153. PMID 15247354.
- ↑ Bjelogrlic SK, Radic J, Radulovic S, Jokanovic M, Jovic V (December 2007). "Effects of dexrazoxane and amifostine on evolution of Doxorubicin cardiomyopathy in vivo". Experimental Biology and Medicine. 232 (11): 1414–24. doi:10.3181/0705-RM-138. PMID 18040065. S2CID 20119026.
- ↑ Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, et al. (February 2007). "Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease". Journal of Clinical Oncology. 25 (5): 493–500. doi:10.1200/JCO.2005.02.3879. PMID 17290056.
- ↑ Salzer WL, Devidas M, Carroll WL, Winick N, Pullen J, Hunger SP, Camitta BA (February 2010). "Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: a report from the children's oncology group". Leukemia. 24 (2): 355–70. doi:10.1038/leu.2009.261. PMC 4300959. PMID 20016527.
- ↑ "FDA Statement on Dexrazoxane". Archived from the original on 2017-01-18. Retrieved 2020-11-05.
- ↑ "Orphan drug designations and approvals". Archived from the original on March 1, 2021. Retrieved June 7, 2019.
- ↑ "Cardioxane". 2018-09-17. Archived from the original on 2020-10-25. Retrieved 2020-11-05.
- ↑ Reichardt P, Tabone MD, Mora J, Morland B, Jones RL (October 2018). "Risk-benefit of dexrazoxane for preventing anthracycline-related cardiotoxicity: re-evaluating the European labeling". Future Oncology. 14 (25): 2663–2676. doi:10.2217/fon-2018-0210. PMID 29747541.
- ↑ "Totect label on FDA's website" (PDF). Archived (PDF) from the original on 2009-03-25. Retrieved 2020-11-05.
- ↑ Kane RC, McGuinn WD, Dagher R, Justice R, Pazdur R (April 2008). "Dexrazoxane (Totect): FDA review and approval for the treatment of accidental extravasation following intravenous anthracycline chemotherapy". The Oncologist. 13 (4): 445–50. doi:10.1634/theoncologist.2007-0247. PMID 18448560.
- ↑ Jones RL (November 2008). "Utility of dexrazoxane for the reduction of anthracycline-induced cardiotoxicity". Expert Review of Cardiovascular Therapy. 6 (10): 1311–7. doi:10.1586/14779072.6.10.1311. PMID 19018683. S2CID 25950994.
- ↑ "ZINECARD- dexrazoxane hydrochloride injection, powder, lyophilized, for solution". Pharmacia and Upjohn Company LLC. Archived from the original on 2021-06-28. Retrieved 2020-11-05.
- ↑ Loyevsky M, Sacci JB, Boehme P, Weglicki W, John C, Gordeuk VR (February 1999). "Plasmodium falciparum and Plasmodium yoelii: effect of the iron chelation prodrug dexrazoxane on in vitro cultures". Experimental Parasitology. 91 (2): 105–14. doi:10.1006/expr.1998.4371. PMID 9990337.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- "Dexrazoxane hydrochloride". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-08-28. Retrieved 2020-11-05.
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Chemicals using indexlabels
- Drugs missing an ATC code
- Drug has EMA link
- Chelating agents
- Chelating agents used as drugs
- Chemotherapeutic adjuvants
- Imides
- Enantiopure drugs
- Diketopiperazines
- RTT