Deucravacitinib
 | |
| Names | |
|---|---|
| Pronunciation | /duːˌkrævəˈsɪtɪnɪb/ doo-KRA-və-SI-ti-nib |
| Trade names | Sotyktu |
| Other names | BMS-986165 |
| |
| Clinical data | |
| Drug class | TYK2 inhibitor[1] |
| Main uses | Plaque psoriasis[1] |
| Side effects | Upper respiratory infections, herpes simplex, folliculitis, acne[1] |
| Routes of use | By mouth |
| Typical dose | 6 mg OD[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 99% |
| Protein binding | 82–90% |
| Metabolism | Liver (primarily CYP1A2) |
| Metabolites | BMT-153261 (active) |
| Elimination half-life | 10 hours |
| Excretion | Feces, urine |
| Chemical and physical data | |
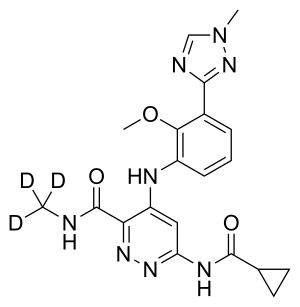
| Formula | C20H22N8O3 |
| Molar mass | 422.449 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Deucravacitinib, sold under the brand name Sotyktu, is a medication used to treat moderate to severe plaque psoriasis.[1] It should not be used with other strong immunosuppressants.[1] It is take by mouth.[1]
Common side effects include upper respiratory infections, herpes simplex, folliculitis, and acne.[1] Other side effects may include allergic reactions, infection, cancer, and muscle breakdown.[1] Use in pregnancy is of unclear safety.[1] Use is not recommended in those with significant liver problems.[1] It is a TYK2 inhibitor.[1]
Deucravacitinib was approved for medical use in the United States in 2022.[1] As of 2022 it is not approved in Europe or the United Kingdom.[2] In the United States it costs about 6,200 USD per month.[2]
Medical use
Dosage
It is take at a dose of 6 mg once per day.[1]
Mechanism of action

It acts as a highly selective allosteric inhibitor of non-receptor tyrosine-protein kinase 2 (TYK2).[4]
Molecule design
The chemical structure of deucravacitinib contains a methyl amide in which all three hydrogen atoms are replaced by deuterium.[5]
History
It was developed by Bristol Myers Squibb.[6]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 "Sotyktu- deucravacitinib tablet, film coated". DailyMed. 9 September 2022. Archived from the original on 28 September 2022. Retrieved 27 September 2022.
- ↑ 2.0 2.1 "Deucravacitinib". SPS - Specialist Pharmacy Service. 1 November 2018. Archived from the original on 29 June 2022. Retrieved 13 December 2022.
- ↑ Alexander, Madison; Luo, Yiming; Raimondi, Giorgio; O'Shea, John J.; Gadina, Massimo (30 December 2021). "Jakinibs of All Trades: Inhibiting Cytokine Signaling in Immune-Mediated Pathologies". Pharmaceuticals (Basel, Switzerland). 15 (1): 48. doi:10.3390/ph15010048. ISSN 1424-8247.
- ↑ Chimalakonda A, Burke J, Cheng L, Catlett I, Tagen M, Zhao Q, et al. (October 2021). "Selectivity Profile of the Tyrosine Kinase 2 Inhibitor Deucravacitinib Compared with Janus Kinase 1/2/3 Inhibitors". Dermatology and Therapy. 11 (5): 1763–1776. doi:10.1007/s13555-021-00596-8. PMC 8484413. PMID 34471993.
- ↑ Mullard A (September 2022). "First de novo deuterated drug poised for approval". Nature Reviews. Drug Discovery. 21 (9): 623–625. doi:10.1038/d41573-022-00139-6. PMID 35974147. S2CID 251623586.
- ↑ "U.S. Food and Drug Administration Approves Sotyktu™ (deucravacitinib), Oral Treatment for Adults with Moderate-to-Severe Plaque Psoriasis". Business Wire (Press release). 10 September 2022. Archived from the original on 10 September 2022. Retrieved 10 September 2022.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Bristol Myers Squibb
- Deuterated compounds
- Non-receptor tyrosine kinase inhibitors
- Triazoles
- Cyclopropyl compounds
- Pyridazines
- Methoxy compounds
- Carboxamides
- RTT