Cobalt(II) phosphate
Jump to navigation
Jump to search
| |

| |
| Names | |
|---|---|
| Other names
cobalt violet, cobalt(II) phosphate, cobalt orthophosphate, Pigment Violet 14
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.309 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Co3(PO4)2 | |
| Molar mass | 366.74231 g/mol |
| Appearance | violet solid |
| Density | 3.81 g/cm3 |
| Melting point | 1,160 °C (2,120 °F; 1,430 K) |
| insoluble | |
Solubility product (Ksp)
|
2.05×10−35[1] |
| 28,110.0·10−6 cm3/mol | |
Refractive index (nD)
|
1.7 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
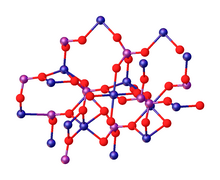
Cobalt phosphate is the inorganic compound with the formula Co3(PO4)2. It is a commercial inorganic pigment known as cobalt violet.[2] Thin films of this material are water oxidation catalysts.[3]

A swatch of cobalt violet, popular among the French impressionists.
Preparation and structure
The tetrahydrate Co3(PO4)2•4H2O precipitates as a solid upon mixing aqueous solutions of cobalt(II) and phosphate salts.[4][5] Upon heating, the tetrahydrate converts to the anhydrous material. According to X-ray crystallography, the anhydrous Co3(PO4)2 consists of discrete phosphate (PO3−
4) anions that link Co2+
centres. The cobalt ions occupy both octahedral (six-coordinate) and pentacoordinate sites in a 1:2 ratio.[6][7]
See also
References
- ^ John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99th ed.). CRC Press. pp. 5–188. ISBN 978-1138561632.
- ^ Hugo Müller, Wolfgang Müller, Manfred Wehner, Heike Liewald "Artists' Colors" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a03_143.pub2
- ^ Matthew W. Kanan; Yogesh Surendranatha; Daniel G. Nocera (2009). "Cobalt–phosphate oxygen-evolving Compound". Chem. Soc. Rev. 38 (1): 109–114. doi:10.1039/B802885K. PMID 19088970.
- ^ Donaldson, John Dallas; Beyersmann, Detmar (2005). "Cobalt and Cobalt Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a07_281.pub2. ISBN 9783527303854.
- ^ Sankar, Selvasundarasekar Sam; Rathishkumar, Arumugam; Geetha, Kathiresan; Kundu, Subrata (2020-10-15). "A Simple Route for the Synthesis of Cobalt Phosphate Nanoparticles for Electrocatalytic Water Oxidation in Alkaline Medium". Energy & Fuels. 34 (10): 12891–12899. doi:10.1021/acs.energyfuels.0c02809. ISSN 0887-0624. S2CID 224960926.
- ^ Anderson, J. B.; Kostiner, E.; Miller, M. C.; Rea, J. R. (1975). "Crystal structure of cobalt orthophosphate Co3(PO4)2". Journal of Solid State Chemistry. 14 (4): 372–377. Bibcode:1975JSSCh..14..372A. doi:10.1016/0022-4596(75)90058-4.
- ^ Nord, A. G.; Stefanidis, T. (1983). "Structure refinements of Co3(PO4)2. A Note on the Reliability of Powder Diffraction Studies". Acta Chemica Scandinavica A. 37: 715–721. doi:10.3891/acta.chem.scand.37a-0715.
