Clostridium botulinum
| Clostridium botulinum | |
|---|---|

| |
| Clostridium botulinum stained with gentian violet. | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Bacillota |
| Class: | Clostridia |
| Order: | Eubacteriales |
| Family: | Lachnospiraceae |
| Genus: | Clostridium |
| Species: | C. botulinum
|
| Binomial name | |
| Clostridium botulinum van Ermengem, 1896
| |
Clostridium botulinum is a Gram-positive,[1] rod-shaped, anaerobic, spore-forming, motile bacterium with the ability to produce the neurotoxin botulinum.[2][3]
The botulinum toxin can cause botulism, a severe flaccid paralytic disease in humans and other animals,[3] and is the most potent toxin known to mankind, natural or synthetic, with a lethal dose of 1.3–2.1 ng/kg in humans.[4]
C. botulinum is a diverse group of pathogenic bacteria initially grouped together by their ability to produce botulinum toxin and now known as four distinct groups, C. botulinum groups I–IV, as well as some strains of Clostridium butyricum and Clostridium baratii, are the bacteria responsible for producing botulinum toxin.[2]
C. botulinum is responsible for foodborne botulism (ingestion of preformed toxin), infant botulism (intestinal infection with toxin-forming C. botulinum), and wound botulism (infection of a wound with C. botulinum). C. botulinum produces heat-resistant endospores that are commonly found in soil and are able to survive under adverse conditions.[2]
C. botulinum is commonly associated with bulging canned food; bulging, misshapen cans can be due to an internal increase in pressure caused by gas produced by bacteria.[5]
Microbiology
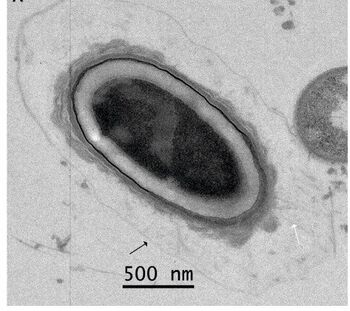
C. botulinum is a Gram-positive, rod-shaped, spore-forming bacterium. It is an obligate anaerobe, meaning that oxygen is poisonous to the cells. However, C. botulinum tolerates traces of oxygen due to the enzyme superoxide dismutase, which is an important antioxidant defense in nearly all cells exposed to oxygen.[6] C. botulinum is able to produce the neurotoxin only during sporulation, which can happen only in an anaerobic environment. C. botulinum is divided into four distinct phenotypic groups (I-IV) and is also classified into seven serotypes (A–G) based on the antigenicity of the botulinum toxin produced.[7][8]
Groups
Physiological differences and genome sequencing at 16S rRNA level support the subdivision of the C. botulinum species into groups I-IV.[9]
| Group | Serotypes |
|---|---|
| I (Proteolytic) | All type A and proteolytic strains of types B and F |
| II (Non proteolytic) | All type E and nonproteolytic strains of types B and F |
| III | Type C and D |
| IV | Type G |
One of the fundamental differences between group I and group II is that C. botulinum group I can lyse native proteins like coagulated egg white, cooked meat particles, whereas group II cannot.[10] However, group II can ferment various carbohydrates like sucrose and mannose, and both of them can degrade the derived protein, gelatin.[10] Human botulism is predominantly caused by group I or II C. botulinum.[10] Group III organisms mainly cause diseases in non-human animals.[10] Group IV C. botulinum has not been shown to cause human or animal disease.[10]
Botulinum toxin
Neurotoxin production is the unifying feature of the species. Eight types of toxins have been identified that are allocated a letter (A–H), several of which can cause disease in humans. They are resistant to degradation by enzymes found in the gastrointestinal tract. This allows for ingested toxins to be absorbed from the intestines into the bloodstream.[4] However, all types of botulinum toxin are rapidly destroyed by heating to 100 °C for 15 minutes (900 seconds). Botulinum toxin, one of the most poisonous biological substances known, is a neurotoxin produced by the bacterium Clostridium botulinum. C. botulinum elaborates eight antigenically distinguishable exotoxins (A, B, C1, C2, D, E, F and G).[11]
Most strains produce one type of neurotoxin, but strains producing multiple toxins have been described. C. botulinum producing B and F toxin types have been isolated from human botulism cases in New Mexico and California.[12] The toxin type has been designated Bf as the type B toxin was found in excess to the type F. Similarly, strains producing Ab and Af toxins have been reported.[citation needed]
Evidence indicates the neurotoxin genes have been the subject of horizontal gene transfer, possibly from a viral (bacteriophage) source. This theory is supported by the presence of integration sites flanking the toxin in some strains of C. botulinum. However, these integrations sites are degraded (except for the C and D types), indicating that the C. botulinum acquired the toxin genes quite far in the evolutionary past. Nevertheless, further transfers still happen via the plasmids and other mobile elements the genes are located on.[13]
Botulinum toxin types
Only botulinum toxin types A, B, E, F and H cause disease in humans. Types A, B, and E are associated with food-borne illness, while type E is specifically associated with fish products. Type C produces limber-neck in birds and type D causes botulism in other mammals. No disease is associated with type G.[14] The "gold standard" for determining toxin type is a mouse bioassay, but the genes for types A, B, E, and F can now be readily differentiated using quantitative PCR.[15] As no antitoxin to type H is yet available, discovered in 2013 and by far the deadliest, details are kept under shroud.[16]
A few strains from organisms genetically identified as other Clostridium species have caused human botulism: C. butyricum has produced type E toxin[17] and C. baratii had produced type F toxin.[18][19] The ability of C. botulinum to naturally transfer neurotoxin genes to other clostridia is concerning, especially in the food industry, where preservation systems are designed to destroy or inhibit only C. botulinum but not other Clostridium species.[citation needed]
| Properties | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Toxin Types | A, B, F | B, E, F | C, D | G |
| Proteolysis | + | – | weak | – |
| Saccharolysis | – | + | – | – |
| Disease host | human | human | animal | – |
| Toxin gene | chromosome/plasmid | chromosome/plasmid | bacteriophage | plasmid |
| Close relatives | C. sporogenes C. putrificum |
C. butyricum C. beijerinickii |
C. haemolyticum C. novyi type A |
C. subterminale C. haemolyticum |
Laboratory isolation
In the laboratory, C. botulinum is usually isolated in tryptose sulfite cycloserine (TSC) growth medium in an anaerobic environment with less than 2% oxygen. This can be achieved by several commercial kits that use a chemical reaction to replace O2 with CO2. C. botulinum is a lipase-positive microorganism that grows between pH of 4.8 and 7.0 and cannot use lactose as a primary carbon source, characteristics important for biochemical identification.[20]
Taxonomy history
C. botulinum was first recognized and isolated in 1895 by Emile van Ermengem from home-cured ham implicated in a botulism outbreak.[21] The isolate was originally named Bacillus botulinus, after the Latin word for sausage, botulus. ("Sausage poisoning" was a common problem in 18th- and 19th-century Germany, and was most likely caused by botulism.)[22] However, isolates from subsequent outbreaks were always found to be anaerobic spore formers, so Ida A. Bengtson proposed that the organism be placed into the genus Clostridium, as the genus Bacillus was restricted to aerobic spore-forming rods.[23]
Since 1959, all species producing the botulinum neurotoxins (types A–G) have been designated C. botulinum. Substantial phenotypic and genotypic evidence exists to demonstrate heterogeneity within the species. This has led to the reclassification of C. botulinum type G strains as a new species, C. argentinense.[24]
Group I C. botulinum strains that do not produce a botulin toxin are referred to as C. sporogenes.[25]
The complete genome of C. botulinum has been sequenced at Wellcome Trust Sanger Institute in 2007.[26]
Pathology
Foodborne botulism
"Signs and symptoms of foodborne botulism typically begin between 18 and 36 hours after the toxin gets into your body, but can range from a few hours to several days, depending on the amount of toxin ingested."[27]
- Double vision
- Blurred vision
- Dropping eyelids
- Nausea, vomiting, and abdominal cramps
- Slurred speech
- Trouble breathing
- Difficulty in swallowing
- Dry mouth
- Muscle weakness
- Constipation
- Reduced or absent deep tendon reactions, such as in the knee
Wound botulism
Most people who develop wound botulism inject drugs several times a day, so it is difficult to determine how long it takes for signs and symptoms to develop after the toxin enters the body. Most common in people who inject black tar heroin, wound botulism signs and symptoms include:[27]
- Difficulty swallowing or speaking
- Facial weakness on both sides of the face
- Blurred or double vision
- Dropping eyelids
- Trouble breathing
- Paralysis
Infant botulism
If infant botulism is related to food, such as honey, problems generally begin within 18 to 36 hours after the toxin enters the baby's body. Signs and symptoms include:[citation needed]
- Constipation (often the first sign)
- Floppy movements due to muscle weakness and trouble controlling the head
- Weak cry
- Irritability
- Drooling
- Dropping eyelids
- Tiredness
- Difficulty sucking or feeding
- Paralysis[27]
Beneficial effects of botulinum toxin
Purified botulinum toxin is diluted by a physician for treatment:
- Congenital pelvic tilt
- Spasmodic dysphasia (the inability of the muscles of the larynx)
- Achalasia (esophageal stricture)
- Strabismus (crossed eyes)
- Paralysis of the facial muscles
- Failure of the cervix
- Blinking frequently
- Anti-cancer drug delivery[28]
Adult intestinal toxemia
A very rare form of botulism that occurs by the same route as infant botulism but is among adults. Occurs rarely and sporadically. Signs and symptoms include:
- Abdominal pain
- Blurred vision
- Diarrhea
- Dysarthria
- Imbalance
- Weakness in arms and hand area[29]
C. botulinum in different geographical locations
A number of quantitative surveys for C. botulinum spores in the environment have suggested a prevalence of specific toxin types in given geographic areas, which remain unexplained.[citation needed]
North America
Type A C. botulinum predominates the soil samples from the western regions, while type B is the major type found in eastern areas.[30] The type-B organisms were of the proteolytic type I. Sediments from the Great Lakes region were surveyed after outbreaks of botulism among commercially reared fish, and only type E spores were detected.[31][32][33] In a survey, type-A strains were isolated from soils that were neutral to alkaline (average pH 7.5), while type-B strains were isolated from slightly acidic soils (average pH 6.23).[citation needed]
Europe
C. botulinum type E is prevalent in aquatic sediments in Norway and Sweden,[34] Denmark,[35] the Netherlands, the Baltic coast of Poland, and Russia.[30] The type-E C. botulinum was suggested to be a true aquatic organism, which was indicated by the correlation between the level of type-E contamination and flooding of the land with seawater. As the land dried, the level of type E decreased and type B became dominant.[citation needed]
In soil and sediment from the United Kingdom, C. botulinum type B predominates. In general, the incidence is usually lower in soil than in sediment. In Italy, a survey conducted in the vicinity of Rome found a low level of contamination; all strains were proteolytic C. botulinum types A or B.[36]
Australia
C. botulinum type A was found to be present in soil samples from mountain areas of Victoria.[37] Type-B organisms were detected in marine mud from Tasmania.[38][verification needed] Type-A C. botulinum has been found in Sydney suburbs and types A and B were isolated from urban areas. In a well-defined area of the Darling-Downs region of Queensland, a study showed the prevalence and persistence of C. botulinum type B after many cases of botulism in horses.[citation needed]
Use and detection
C. botulinum is used to prepare the medicaments Botox, Dysport, Xeomin, and Neurobloc used to selectively paralyze muscles to temporarily relieve muscle function. It has other "off-label" medical purposes, such as treating severe facial pain, such as that caused by trigeminal neuralgia.[citation needed]
Botulinum toxin produced by C. botulinum is often believed to be a potential bioweapon as it is so potent that it takes about 75 nanograms to kill a person (LD50 of 1 ng/kg,[39] assuming an average person weighs ~75 kg); 1 kilogram of it would be enough to kill the entire human population. For comparative purposes, a quarter of a typical grain of sand's weight (350 ng) of botulinum toxin would constitute a lethal dose for humans.[citation needed]
A "mouse protection" or "mouse bioassay" test determines the type of C. botulinum toxin present using monoclonal antibodies. An enzyme-linked immunosorbent assay (ELISA) with digoxigenin-labeled antibodies can also be used to detect the toxin,[40] and quantitative PCR can detect the toxin genes in the organism.[15]
Growth conditions and prevention
C. botulinum is a soil bacterium. The spores can survive in most environments and are very hard to kill. They can survive the temperature of boiling water at sea level, thus many foods are canned with a pressurized boil that achieves even higher temperatures, sufficient to kill the spores.[41][42] This bacteria is widely distributed in nature and can be assumed to be present on all food surfaces. Its optimum growth temperature is within the mesophilic range. In spore form, it is a heat resistant pathogen that can survive in low acid foods and grow to produce toxins. The toxin attacks the nervous system and will kill an adult at a dose of around 75 ng.[39] This toxin is detoxified by holding food at 100 °C for 10 minutes.[43]
Botulism poisoning can occur due to preserved or home-canned, low-acid food that was not processed using correct preservation times and/or pressure.[44] Growth of the bacterium can be prevented by high acidity, high ratio of dissolved sugar, high levels of oxygen, very low levels of moisture, or storage at temperatures below 3 °C (38 °F) for type A. For example, in a low-acid, canned vegetable such as green beans that are not heated enough to kill the spores (i.e., a pressurized environment) may provide an oxygen-free medium for the spores to grow and produce the toxin. However, pickles are sufficiently acidic to prevent growth;[non-primary source needed] even if the spores are present, they pose no danger to the consumer.
Honey, corn syrup, and other sweeteners may contain spores, but the spores cannot grow in a highly concentrated sugar solution; however, when a sweetener is diluted in the low-oxygen, low-acid digestive system of an infant, the spores can grow and produce toxin. As soon as infants begin eating solid food, the digestive juices become too acidic for the bacterium to grow.[45]
The control of food-borne botulism caused by C. botulinum is based almost entirely on thermal destruction (heating) of the spores or inhibiting spore germination into bacteria and allowing cells to grow and produce toxins in foods. Conditions conducive of growth are dependent on various environmental factors. Growth of C. botulinum is a risk in low acid foods as defined by having a pH above 4.6[46] although growth is significantly retarded for pH below 4.9.[citation needed]
In the beginning of 21st century there have been some cases and specific conditions reported to sustain growth with pH below 4.6. but at higher temperature.[47][48]
Diagnosis
Physicians may consider the diagnosis of botulism based on a patient's clinical presentation, which classically includes an acute onset of bilateral cranial neuropathies and symmetric descending weakness.[49][50] Other key features of botulism include an absence of fever, symmetric neurologic deficits, normal or slow heart rate and normal blood pressure, and no sensory deficits except for blurred vision.[51][52] A careful history and physical examination is paramount in order to diagnose the type of botulism, as well as to rule out other conditions with similar findings, such as Guillain–Barré syndrome, stroke, and myasthenia gravis.[citation needed] Depending on the type of botulism considered, different tests for diagnosis may be indicated.
- Foodborne botulism: serum analysis for toxins by bioassay in mice should be done, as the demonstration of the toxins is diagnostic.[53]
- Wound botulism: isolation of C. botulinum from the wound site should be attempted, as growth of the bacteria is diagnostic.[54]
- Adult enteric and infant botulism: isolation and growth of C. botulinum from stool samples is diagnostic.[55] Infant botulism is a diagnosis which is often missed in the emergency room.[citation needed]
Other tests that may be helpful in ruling out other conditions are:
- Electromyography (EMG) or antibody studies may help with the exclusion of myasthenia gravis and Lambert–Eaton myasthenic syndrome (LEMS).[56]
- Collection of cerebrospinal fluid (CSF) protein and blood assist with the exclusion of Guillan-Barre syndrome and stroke.[57]
- Detailed physical examination of the patient for any rash or tick presence helps with the exclusion of any tick transmitted tick paralysis.[58]
Treatment
In the case of a diagnosis or suspicion of botulism, patients should be hospitalized immediately, even if the diagnosis and/or tests are pending. If botulism is suspected, patients should be treated immediately with antitoxin therapy in order to reduce mortality. Immediate intubation is also highly recommended, as respiratory failure is the primary cause of death from botulism.[59][60][61]
In Canada, there are currently only three antitoxin therapies available, which are accessible through Health Canada Special Access Program (SAP).[62] The three types of antitoxin therapies are: 1) GlaxoSmithKline trivalent Types ABE, 2) NP-018 (heptavalent) Types A to G, and 3) BabyBIG, Botulism Immune Globulin Intravenous (Human) (BIG-IV) for pediatric patients under the age of one year.[62]
Outcomes vary between one and three months, but with prompt interventions, mortality from botulism ranges from less than 5 percent to 8 percent.[63]
Vaccination
There used to be a formalin-treated toxoid vaccine against botulism (serotypes A-E), but it was discontinued in 2011 due to declining potency in the toxoid stock. It was originally intended for people at risk of exposure. A few new vaccines are under development.[64]
References
- ↑ Tiwari, Aman; Nagalli, Shivaraj (2021), "Clostridium Botulinum", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 31971722, archived from the original on 2020-12-27, retrieved 2021-09-23
- ↑ 2.0 2.1 2.2 Peck MW (2009). Biology and Genomic Analysis of Clostridium botulinum. Advances in Microbial Physiology. Advances in Microbial Physiology. Vol. 55. pp. 183–265, 320. doi:10.1016/s0065-2911(09)05503-9. ISBN 9780123747907. PMID 19573697.
- ↑ 3.0 3.1 Lindström M, Korkeala H (April 2006). "Laboratory diagnostics of botulism". Clinical Microbiology Reviews. 19 (2): 298–314. doi:10.1128/cmr.19.2.298-314.2006. PMC 1471988. PMID 16614251.
- ↑ 4.0 4.1 (2010). Chapter 19. Clostridium, Peptostreptococcus, Bacteroides, and Other Anaerobes. In Ryan K.J., Ray C (Eds), Sherris Medical Microbiology, 5th ed. ISBN 978-0-07-160402-4
- ↑ Schneider KR, Silverberg R, Chang A, Goodrich Schneider RM (9 January 2015). "Preventing Foodborne Illness: Clostridium botulinum". edis.ifas.ufl.edu. University of Florida IFAS Extension. Archived from the original on 8 February 2017. Retrieved 7 February 2017.
- ↑ Doyle MP (2007). Food Microbiology: Fundamentals and Frontiers. ASM Press. ISBN 978-1-55581-208-9.
- ↑ Peck MW, Stringer SC, Carter AT (April 2011). "Clostridium botulinum in the post-genomic era". Food Microbiology. 28 (2): 183–91. doi:10.1016/j.fm.2010.03.005. PMID 21315972.
- ↑ Shukla HD, Sharma SK (2005). "Clostridium botulinum: a bug with beauty and weapon". Critical Reviews in Microbiology. 31 (1): 11–8. doi:10.1080/10408410590912952. PMID 15839401. S2CID 2855356.
- ↑ 9.0 9.1 Austin, J.W. (January 1, 2003). "CLOSTRIDIUM | Occurrence of Clostridium botulinum". CLOSTRIDIUM. ScienceDirect. Academic Press. pp. 1407–1413. doi:10.1016/B0-12-227055-X/00255-8. ISBN 9780122270550. Archived from the original on March 14, 2022. Retrieved February 19, 2021.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Carter, Andrew T.; Peck, Michael W. (October 18, 1957). "Genomes, neurotoxins and biology of Clostridium botulinum Group I and Group II". Research in Microbiology. 166 (4): 303–317. doi:10.1016/j.resmic.2014.10.010. PMC 4430135. PMID 25445012.
- ↑ Montecucco C, Molgó J (June 2005). "Botulinal neurotoxins: revival of an old killer". Current Opinion in Pharmacology. 5 (3): 274–9. doi:10.1016/j.coph.2004.12.006. PMID 15907915.
- ↑ Hatheway CL, McCroskey LM (December 1987). "Examination of feces and serum for diagnosis of infant botulism in 336 patients". Journal of Clinical Microbiology. 25 (12): 2334–8. doi:10.1128/JCM.25.12.2334-2338.1987. PMC 269483. PMID 3323228.
- ↑ Poulain B, Popoff MR (January 2019). "Why Are Botulinum Neurotoxin-Producing Bacteria So Diverse and Botulinum Neurotoxins So Toxic?". Toxins. 11 (1): 34. doi:10.3390/toxins11010034. PMC 6357194. PMID 30641949.
- ↑ (2013). Chapter 11. Spore-Forming Gram-Positive Bacilli: Bacillus and Clostridium Species. In Brooks G.F., Carroll K.C., Butel J.S., Morse S.A., Mietzner T.A. (Eds), Jawetz, Melnick, & Adelberg's Medical Microbiology, 26th ed. ISBN 978-0-07-179031-4
- ↑ 15.0 15.1 Satterfield BA, Stewart AF, Lew CS, Pickett DO, Cohen MN, Moore EA, et al. (January 2010). "A quadruplex real-time PCR assay for rapid detection and differentiation of the Clostridium botulinum toxin genes A, B, E and F". Journal of Medical Microbiology. 59 (Pt 1): 55–64. doi:10.1099/jmm.0.012567-0. PMID 19779029.
- ↑ "Botulinum Toxin type H – the Deadliest Known Toxin with no Known Antidote Discovered". Nature World News. October 15, 2013. Archived from the original on April 17, 2018. Retrieved April 13, 2018.
- ↑ Aureli P, Fenicia L, Pasolini B, Gianfranceschi M, McCroskey LM, Hatheway CL (August 1986). "Two cases of type E infant botulism caused by neurotoxigenic Clostridium butyricum in Italy". The Journal of Infectious Diseases. 154 (2): 207–11. doi:10.1093/infdis/154.2.207. PMID 3722863.
- ↑ Hall JD, McCroskey LM, Pincomb BJ, Hatheway CL (April 1985). "Isolation of an organism resembling Clostridium barati which produces type F botulinal toxin from an infant with botulism". Journal of Clinical Microbiology. 21 (4): 654–5. doi:10.1128/JCM.21.4.654-655.1985. PMC 271744. PMID 3988908.
- ↑ Notermans S, Havellar AH (1980). "Removal and inactivation of botulinum toxin during production of drinking water from surface water". Antonie van Leeuwenhoek. 46 (5): 511–514. doi:10.1007/BF00395840. S2CID 21102990.
- ↑ Madigan MT, Martinko JM, eds. (2005). Brock Biology of Microorganisms (11th ed.). Prentice Hall. ISBN 978-0-13-144329-7.
- ↑ van Ergmengem E (1897). "Über einen neuen anaeroben Bacillus und seine Beziehungen Zum Botulismus". Zeitschrift für Hygiene und Infektionskrankheiten. 26: 1–8.
- ↑ Erbguth FJ (March 2004). "Historical notes on botulism, Clostridium botulinum, botulinum toxin, and the idea of the therapeutic use of the toxin". Movement Disorders. 19 (Suppl 8): S2-6. doi:10.1002/mds.20003. PMID 15027048. S2CID 8190807.
- ↑ Bengston IA (1924). "Studies on organisms concerned as causative factors in botulism". Bulletin (Hygienic Laboratory (U.S.)). 136: 101 fv.
- ↑ Suen JC, Hatheway CL, Steigerwalt AG, Brenner DJ (1988). "Clostridium argentinense sp.nov.: a genetically homogeneous group composed of all strains of Clostridium botulinum type G and some nontoxigenic strains previously identified as Clostridium subterminale or Clostridium hastiforme". International Journal of Systematic Bacteriology. 38: 375–381. doi:10.1099/00207713-38-4-375.
- ↑ "Rejection of Clostridium putrificum and conservation of Clostridium botulinum and Clostridium sporogenes-Opinion 69. Judicial Commission of the International Committee on Systematic Bacteriology". International Journal of Systematic Bacteriology. 49 (1): 339. January 1999. doi:10.1099/00207713-49-1-339. PMID 10028279.
- ↑ "Clostridium botulinum". Wellcome Trust Sanger Institute. 2007. Archived from the original on March 20, 2016. Retrieved January 25, 2016.
- ↑ 27.0 27.1 27.2 "Botulism Symptoms". Mayo Clinic. June 13, 2015. Archived from the original on January 15, 2016. Retrieved January 25, 2016.
- ↑ Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, et al. (February 2001). "Botulinum toxin as a biological weapon: medical and public health management". JAMA. 285 (8): 1059–70. doi:10.1001/jama.285.8.1059. PMID 11209178.
- ↑ "Botulism". Centers for Disease Control and Prevention. 2016. 23 October 2016.
- ↑ 30.0 30.1 Hauschild, A. H. W. (1989). "Clostridium botulinum". In Doyle, M. P. (ed.). Food-borne Bacterial Pathogens. New York: Marcel Dekker. pp. 111–189. ISBN 0-8247-7866-9.
- ↑ Bott TL, Johnson J, Foster EM, Sugiyama H (May 1968). "Possible origin of the high incidence of Clostridium botulinum type E in an inland bay (Green Bay of Lake Michigan)". Journal of Bacteriology. 95 (5): 1542–7. doi:10.1128/JB.95.5.1542-1547.1968. PMC 252172. PMID 4870273.
- ↑ Eklund MW, Peterson ME, Poysky FT, Peck LW, Conrad JF (1982). "Botulism in juvenile Coho salmon (Onocorhynchus kisutch) in the United States". Aquaculture. 27: 1–11. doi:10.1016/0044-8486(82)90104-1.
- ↑ Eklund MW, Poysky FT, Peterson ME, Peck LW, Brunson WD (1984). "Type E botulism in salmonids and conditions contributing to outbreaks". Aquaculture. 41 (4): 293–309. doi:10.1016/0044-8486(84)90198-4.
- ↑ Johannsen, A. (1963). "Clostridium botulinum in Sweden and the adjacent waters". Journal of Applied Microbiology. 26 (1): 43–47. doi:10.1111/j.1365-2672.1963.tb01153.x.
- ↑ Huss HH (April 1980). "Distribution of Clostridium botulinum". Applied and Environmental Microbiology. 39 (4): 764–9. Bibcode:1980ApEnM..39..764H. doi:10.1128/AEM.39.4.764-769.1980. PMC 291416. PMID 6990867.
- ↑ Creti R, Fenicia L, Aureli P (1990). "Occurrence of Clostridium botulinum in the soil of the vicinity of Rome". Current Microbiology. 20 (5): 317. doi:10.1007/bf02091912. S2CID 44379501.
- ↑ Eales CE, Gillespie JM (August 1947). "The isolation of Clostridium botulinum type A from Victorian soils". The Australian Journal of Science. 10 (1): 20. PMID 20267540.
- ↑ Ohye WJ (1957). "Studies in the physiology of Clostridium botulinum type E." Australian Journal of Biological Sciences. 10: 85–94. doi:10.1071/BI9570085.
- ↑ 39.0 39.1 Fleming DO. Biological Safety: principles and practices. Vol. 2000. ASM Press. p. 267.
- ↑ Sharma SK, Ferreira JL, Eblen BS, Whiting RC (February 2006). "Detection of type A, B, E, and F Clostridium botulinum neurotoxins in foods by using an amplified enzyme-linked immunosorbent assay with digoxigenin-labeled antibodies". Applied and Environmental Microbiology. 72 (2): 1231–8. Bibcode:2006ApEnM..72.1231S. doi:10.1128/AEM.72.2.1231-1238.2006. PMC 1392902. PMID 16461671.
- ↑ CDC (2019-06-06). "Prevent Botulism". Centers for Disease Control and Prevention. Archived from the original on 2023-04-23. Retrieved 2023-04-23.
- ↑ "Botulism: take care when canning low-acid foods". extension.umn.edu. Archived from the original on 2023-04-23. Retrieved 2023-04-23.
- ↑ "CHAPTER 13: Clostridium botulinum Toxin Formation" (PDF). Fda.gov. Archived (PDF) from the original on 2021-02-08. Retrieved 18 March 2022.
- ↑ "Home Canning and Botulism". Centers for Disease Control and Prevention. Archived from the original on 2 August 2022. Retrieved 14 April 2021.
- ↑ "Botulism". The Lecturio Medical Concept Library. Archived from the original on 9 July 2021. Retrieved 5 July 2021.
- ↑ "Guidance for Commercial Processors of Acidified & Low-Acid Canned Foods". U.S. Food and Drug Administration. Archived from the original on 7 October 2016. Retrieved 8 October 2016.
- ↑ Lund BM, Graham AF, Franklin JG (June 1987). "The effect of acid pH on the probability of growth of proteolytic strains of Clostridium botulinum". International Journal of Food Microbiology. 4 (3): 215–226. doi:10.1016/0168-1605(87)90039-0.
- ↑ Raatjes GJ, Smelt JP (October 1979). "Clostridium botulinum can grow and form toxin at pH values lower than 4.6". Nature. 281 (5730): 398–9. Bibcode:1979Natur.281..398R. doi:10.1038/281398a0. PMID 39257. S2CID 4360451.
- ↑ Cherington M (June 1998). "Clinical spectrum of botulism". Muscle & Nerve. 21 (6): 701–10. doi:10.1002/(sici)1097-4598(199806)21:6<701::aid-mus1>3.0.co;2-b. PMID 9585323. S2CID 12611619.
- ↑ Cai S, Singh BR, Sharma S (April 2007). "Botulism diagnostics: from clinical symptoms to in vitro assays". Critical Reviews in Microbiology. 33 (2): 109–25. doi:10.1080/10408410701364562. PMID 17558660. S2CID 23470999.
- ↑ "Diagnosis and Treatment | Botulism". CDC. Archived from the original on 2016-10-05. Retrieved 2017-10-08.
- ↑ "Botulism: Rare but serious food poisoning". Mayo Clinic. Archived from the original on 2017-09-22. Retrieved 2017-11-18.
- ↑ Lindström M, Korkeala H (April 2006). "Laboratory diagnostics of botulism". Clinical Microbiology Reviews. 19 (2): 298–314. doi:10.1128/CMR.19.2.298-314.2006. PMC 1471988. PMID 16614251.
- ↑ Akbulut D, Grant KA, McLauchlin J (September 2005). "Improvement in laboratory diagnosis of wound botulism and tetanus among injecting illicit-drug users by use of real-time PCR assays for neurotoxin gene fragments". Journal of Clinical Microbiology. 43 (9): 4342–8. doi:10.1128/JCM.43.9.4342-4348.2005. PMC 1234055. PMID 16145075.
- ↑ Dezfulian M, McCroskey LM, Hatheway CL, Dowell VR (March 1981). "Selective medium for isolation of Clostridium botulinum from human feces". Journal of Clinical Microbiology. 13 (3): 526–31. doi:10.1128/JCM.13.3.526-531.1981. PMC 273826. PMID 7016901.
- ↑ O'Suilleabhain P, Low PA, Lennon VA (January 1998). "Autonomic dysfunction in the Lambert-Eaton myasthenic syndrome: serologic and clinical correlates". Neurology. 50 (1): 88–93. doi:10.1212/wnl.50.1.88. PMID 9443463. S2CID 39437882. Archived from the original on 2023-02-12. Retrieved 2023-04-23.
- ↑ Mechem CC, Walter FG (June 1994). "Wound botulism". Veterinary and Human Toxicology. 36 (3): 233–7. PMID 8066973. Archived from the original on 2023-02-12. Retrieved 2023-04-23.
- ↑ Taraschenko OD, Powers KM (June 2014). "Neurotoxin-induced paralysis: a case of tick paralysis in a 2-year-old child". Pediatric Neurology. 50 (6): 605–7. doi:10.1016/j.pediatrneurol.2014.01.041. PMID 24679414.
- ↑ Witoonpanich R, Vichayanrat E, Tantisiriwit K, Wongtanate M, Sucharitchan N, Oranrigsupak P, et al. (March 2010). "Survival analysis for respiratory failure in patients with food-borne botulism". Clinical Toxicology. 48 (3): 177–83. doi:10.3109/15563651003596113. PMID 20184431. S2CID 23108891.
- ↑ Sandrock CE, Murin S (August 2001). "Clinical predictors of respiratory failure and long-term outcome in black tar heroin-associated wound botulism". Chest. 120 (2): 562–6. doi:10.1378/chest.120.2.562. PMID 11502659.
- ↑ Wongtanate M, Sucharitchan N, Tantisiriwit K, Oranrigsupak P, Chuesuwan A, Toykeaw S, Suputtamongkol Y (August 2007). "Signs and symptoms predictive of respiratory failure in patients with foodborne botulism in Thailand". The American Journal of Tropical Medicine and Hygiene. 77 (2): 386–9. doi:10.4269/ajtmh.2007.77.386. PMID 17690419.
- ↑ 62.0 62.1 "Botulism – Guide for Healthcare Professionals". Canada Health. 18 July 2012. Archived from the original on 2017-10-19. Retrieved 2017-10-08.
- ↑ Varma JK, Katsitadze G, Moiscrafishvili M, Zardiashvili T, Chokheli M, Tarkhashvili N, et al. (August 2004). "Signs and symptoms predictive of death in patients with foodborne botulism--Republic of Georgia, 1980-2002". Clinical Infectious Diseases. 39 (3): 357–62. doi:10.1086/422318. PMID 15307002.
- ↑ Sundeen G, Barbieri JT (September 2017). "Vaccines against Botulism". Toxins. 9 (9): 268. doi:10.3390/toxins9090268. PMC 5618201. PMID 28869493.
External links
- Sobel J (October 2005). "Botulism". Clinical Infectious Diseases. 41 (8): 1167–73. doi:10.1086/444507. PMID 16163636.
- Articles with 'species' microformats
- All articles with unsourced statements
- Articles with unsourced statements from January 2021
- Articles with invalid date parameter in template
- Articles with unsourced statements from June 2022
- All pages needing factual verification
- Wikipedia articles needing factual verification from January 2017
- Articles with unsourced statements from December 2022
- Wikipedia articles needing factual verification from October 2021
- Articles with unsourced statements from April 2023
- Articles with unsourced statements from February 2023
- Articles with unsourced statements from January 2023
- Bacteria described in 1896
- Botulism
- Clostridium
- Food microbiology
- Gram-positive bacteria