Balsalazide
 | |
| Names | |
|---|---|
| Trade names | Colazide,[1] Giazo, Colazal[2] |
| Other names | Balsalazide sodium, balsalazide disodium[1] |
| |
| Clinical data | |
| Drug class | Prodrug of mesalamine[3] |
| Main uses | Ulcerative colitis, Crohn’s disease[1][3] |
| Side effects | Headache, feeling sick, stomach upset, diarrhea, joint pain, respiratory infections[3] |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699052 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | <1% |
| Protein binding | ≥99% |
| Elimination half-life | 12hr |
| Chemical and physical data | |
| Formula | C17H15N3O6 |
| Molar mass | 357.322 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Balsalazide, sold under the brand name Colazide among others, is a medication used to treat ulcerative colitis (UC) and Crohn’s disease.[1][3] In UC it is used for mild to moderate disease and usually only for up to 12 weeks.[3] It is take by mouth.[1]
Common side effects include headache, feeling sick, stomach upset, diarrhea, joint pain, and respiratory infections.[3] Other side effects may include blood disorder, gallstones, or a lupus-like syndrome.[1] Safety in pregnancy is unclear.[6] An alternative may be required for people with asthma, kidney or liver problems.[1] Balsalazide passes unchanged through the stomach and small bowel, and releases mesalazine (5-ASA) when it reaches the large bowel, where ulcerative colitis affects.[7]
Balsalazide was first made in the 1980s and approved for medical use in the United Kingdom in 1997.[8] It is available as a generic medication.[9] In the United Kingdom a months supply at the maintenance dose costs the NHS around £30 as of 2021.[1] In the United States this amount costs about 74 USD.[10]
Medical use
Balsalazide is used to treat mild to moderate ulcerative colitis and to prevent acute attacks.[1] A blood test to check for kidney problems is required before starting balsalazide.[5]
Dose
An adult dose is typically 2.25g three times a day until symptoms settle, or for up to a maximum of 12 weeks.[1] Once settled, a maintenance dose is typically 1.5g twice a day, but can be more if required.[1] The maximum dose in adults is 6g in one day.[1] The Colazal form may be given to children over 5 years old.[2] It can be taken with or without food, and if unable to swallow the capsule, its contents can be sprinkled on a spoon of applesauce and taken immediately.[2]
Availabilty
Colazal and Colazide are available as 750mg capsules.[1][2] The Giazo brand comes in a 1.1g tablet.[2] In the US, Colazal can be used in children over age 5-years, and Giazo brand for use in men over the age of 18 years, but for a maximum of eight weeks duration.[1][2]
Side effects
An alternative may be required for people with asthma, kidney or liver problems.[1] Its effects in pregnancy are not known and it is generally not used in pregnancy.[2][5] Side effects include headache, feeling sick, stomach upset, diarrhea, joint pain, fever, sore throat, and runny nose.[2] Balsalazide may cause blood disorder, gallstones or lupus-like syndrome; which may cause tiredness, pale skin, feeling dizzy or short of breath.[1][2]
Mechanism
Balsalazide is a type of azo compound belonging to the aminosalicylates.[7] The mesalazine (5-ASA) in balsalazide is bound by an azo (N=N) bond to the inert (4-aminobenzoyl)-beta-alanine.[7] This azo-structure reduces absorption of balsalazide from the small intestine.[7] Once taken by mouth, balsalazide passes unchanged through the stomach and small bowel, but when it reaches azoreductase-producing bacteria in the large bowel, the azo bond is broken and 5-ASA is released at the site of the ulcerative colitis.[7] A dose of 6.75g of balsalazide is equivalent to 2.4g of mesalazine.[11] It is unclear how mesalazine works.[11]
Synthesis
Ex 3 is actually for Ipsalazide. See Ex 4 for Balsalazide proper. Same protocol but uses β-Alanine.
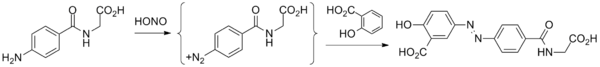
- Starting material is 4-aminohippuric acid, obtained by coupling para-aminobenzoic acid and glycine.
- That product is then treated with nitrous acid to give the diazonium salt.
- Reaction of this species with salicylic acid proceeds at the position para to the phenol to give balsalazide.
History
Balsalazide was first produced in the 1980s by Biorex Laboratories and marketed as Colazal first in the UK in 1996.[8]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 "1. Gastro-intestinal system". British National Formulary (BNF) (82 ed.). London: BMJ Group and the Pharmaceutical Press. September 2021 – March 2022. pp. 44–45. ISBN 978-0-85711-413-6.
{{cite book}}: CS1 maint: date format (link) - ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "A - Z Drug List from Drugs.com: Balsalazide". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 22 November 2021. Retrieved 22 November 2021.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "Balsalazide Monograph for Professionals". Drugs.com. Archived from the original on 18 December 2021. Retrieved 8 January 2022.
- ↑ "Balsalazide Use During Pregnancy". Drugs.com. Archived from the original on 1 December 2020. Retrieved 22 November 2021.
- ↑ 5.0 5.1 5.2 "Colazide 750mg Capsules - Summary of Product Characteristics (SmPC)". (emc). 3 January 2019. Archived from the original on 22 January 2021. Retrieved 2 October 2020.
- ↑ "Balsalazide Use During Pregnancy". Drugs.com. Archived from the original on 1 December 2020. Retrieved 8 January 2022.
- ↑ 7.0 7.1 7.2 7.3 7.4 McQuaid, Kenneth R. (2020). "62. Drugs used in the treatment of gastrointestinal diseases". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. pp. 1150–1151. ISBN 978-1-260-45231-0. Archived from the original on 2021-10-10. Retrieved 2021-11-22.
- ↑ 8.0 8.1 Richmond, Lesley; Stevenson, Julie (2017). The Pharmaceutical Industry: A Guide to Historical Records. Routledge. p. 135. ISBN 978-1-351-88429-7. Archived from the original on 2021-12-11. Retrieved 2021-11-23.
- ↑ "Balsalazide Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 23 October 2016. Retrieved 8 January 2022.
- ↑ "Balsalazide Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 8 January 2022.
- ↑ 11.0 11.1 Shargel, Leon; Kanfer, Isadore (2016). Generic Drug Product Development: Specialty Dosage Forms. CRC Press. ISBN 978-1-4200-2003-8. Archived from the original on 2021-12-11. Retrieved 2021-11-22.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- CS1 maint: date format
- Drugs with non-standard pregnancy category
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Chemicals using indexlabels
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Articles with changed EBI identifier
- Gastroenterology
- Azo compounds
- Salicylic acids
- Benzamides
- Anilines
- Propionic acids
- RTT