Avacopan
 | |
| Names | |
|---|---|
| Trade names | Tavneos, Vynpenta |
| Other names | CCX168 |
| Clinical data | |
| Drug class | Complement C5a receptor antagonist |
| Main uses | Granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA)[1] |
| Side effects | Nausea, headache, low white blood cells, upper respiratory tract infection, diarrhea, vomiting, inflammation of the nose and throat[1] |
| Routes of use | By mouth |
| Typical dose | 30 mg BID[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
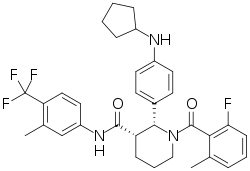
| Formula | C33H35F4N3O2 |
| Molar mass | 581.656 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Avacopan, sold under the brand name Tavneos, is a medication used to treat anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis.[2] This includes granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).[1] It is taken by mouth.[2]
Common side effects include nausea, headache, low white blood cells, upper respiratory tract infection, diarrhea, vomiting, and inflammation of the nose and throat.[1] Other side effects may include liver problems, allergic reactions, and infections.[2] Safety is unclear in pregnancy.[2] It is a complement 5a receptor (C5aR) antagonist.[2]
Avacopan was approved for medical use in Japan and United States in 2021, and Europe in 2022.[4][2][1] In the United Kingdom a month of medication costs the NHS about £5500 as of 2022.[5] This amount in the United States costs about 14,400 USD.[6]
Medical uses
In the United States, it is used as an adjunctive treatment of adults with severe active anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (granulomatosis with polyangiitis and microscopic polyangiitis) in combination with standard therapy including glucocorticoids.[2][7]
In the European Union, avacopan, in combination with a rituximab or cyclophosphamide regimen, is indicated for the treatment of adults with severe, active granulomatosis with polyangiitis or microscopic polyangiitis (MPA).[1]
Dosage
It is generally used at a dose of 30 mg twice per day.[2]
Mechanism of action

In terms of the mechanism of action we find that Avacopan works by targeting and blocking the complement 5a receptor, as a consequence this prevents the activation of neutrophils, reducing inflammation, as well as damage to blood vessels[8]
History
It is the first orally-administered inhibitor of the complement C5a receptor approved in the USA.[9]
Society and culture
Legal status
In November 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Tavneos, intended, in combination with a rituximab or cyclophosphamide regimen, for the treatment of adults with severe, active granulomatosis with polyangiitis or microscopic polyangiitis.[10] The applicant for this medicinal product is Vifor Fresenius Medical Care Renal Pharma France.[10] The EMA considers avacopan to be a first-in-class medicine.[11] Avacopan was approved for medical use in the European Union in January 2022.[1]
Names
Avacopan is the international nonproprietary name (INN).[12]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Tavneos EPAR". European Medicines Agency. 10 November 2021. Archived from the original on 17 March 2022. Retrieved 24 April 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 "Tavneos- avacopan capsule". DailyMed. Archived from the original on 1 November 2021. Retrieved 31 October 2021.
- ↑ "Summary Basis of Decision - Tavneos". Health Canada. 28 July 2022. Archived from the original on 29 September 2022. Retrieved 29 September 2022.
- ↑ 4.0 4.1 "ChemoCentryx Announces Approval in Japan of Tavneos (Avacopan) for the Treatment of ANCA-Associated Vasculitis". ChemoCentryx, Inc. (Press release). 27 September 2021. Archived from the original on 9 October 2021. Retrieved 11 October 2021.
- ↑ "Avacopan". SPS - Specialist Pharmacy Service. 23 October 2017. Archived from the original on 6 December 2021. Retrieved 25 October 2022.
- ↑ "Avacopan". Goodrx. Archived from the original on 25 October 2022. Retrieved 25 October 2022.
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214487Orig1s000Approv.pdf Archived 7 May 2022 at the Wayback Machine[bare URL PDF]
- ↑ 8.0 8.1 Paroli, Marino; Gioia, Chiara; Accapezzato, Daniele (March 2023). "New Insights into Pathogenesis and Treatment of ANCA-Associated Vasculitis: Autoantibodies and Beyond". Antibodies. 12 (1): 25. doi:10.3390/antib12010025. ISSN 2073-4468. Archived from the original on 11 April 2024. Retrieved 15 April 2024.
- ↑ "ChemoCentryx Announces FDA Approval of Tavneos (avacopan) in ANCA-Associated Vasculitis". ChemoCentryx, Inc. (Press release). 8 October 2021. Archived from the original on 8 October 2021. Retrieved 11 October 2021.
- ↑ 10.0 10.1 "Tavneos: Pending EC decision". European Medicines Agency. 11 November 2021. Archived from the original on 12 November 2021. Retrieved 12 November 2021. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "First-in-class medicine recommended for treatment of rare blood vessel inflammation". European Medicines Agency (Press release). 12 November 2021. Archived from the original on 12 November 2021. Retrieved 12 November 2021.
- ↑ World Health Organization (2016). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 76". WHO Drug Information. 30 (3). hdl:10665/331020.
Further reading
- Jayne DR, Bruchfeld AN, Harper L, Schaier M, Venning MC, Hamilton P, et al. (September 2017). "Randomized Trial of C5a Receptor Inhibitor Avacopan in ANCA-Associated Vasculitis". J Am Soc Nephrol. 28 (9): 2756–2767. doi:10.1681/ASN.2016111179. PMC 5576933. PMID 28400446.
- Jayne DR, Merkel PA, Schall TJ, Bekker P (February 2021). "Avacopan for the Treatment of ANCA-Associated Vasculitis". N Engl J Med. 384 (7): 599–609. doi:10.1056/NEJMoa2023386. PMID 33596356.
- Merkel PA, Jayne DR, Wang C, Hillson J, Bekker P (April 2020). "Evaluation of the Safety and Efficacy of Avacopan, a C5a Receptor Inhibitor, in Patients With Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Treated Concomitantly With Rituximab or Cyclophosphamide/Azathioprine: Protocol for a Randomized, Double-Blind, Active-Controlled, Phase 3 Trial". JMIR Res Protoc. 9 (4): e16664. doi:10.2196/16664. PMC 7175182. PMID 32088663.
- Merkel PA, Niles J, Jimenez R, Spiera RF, Rovin BH, Bomback A, et al. (November 2020). "Adjunctive Treatment With Avacopan, an Oral C5a Receptor Inhibitor, in Patients With Antineutrophil Cytoplasmic Antibody-Associated Vasculitis". ACR Open Rheumatol. 2 (11): 662–671. doi:10.1002/acr2.11185. PMC 7672305. PMID 33128347.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Clinical trial number NCT02994927 for "A Phase 3 Clinical Trial of CCX168 (Avacopan) in Patients With ANCA-Associated Vasculitis (ADVOCATE)" at ClinicalTrials.gov
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- All articles with bare URLs for citations
- Articles with bare URLs for citations from May 2022
- Articles with invalid date parameter in template
- Articles with PDF format bare URLs for citations
- Use American English from October 2021
- All Wikipedia articles written in American English
- Use dmy dates from September 2022
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Drugs not assigned an ATC code
- Orphan drugs
- Fluoroarenes
- Trifluoromethyl compounds
- Amides
- Anilines
- Piperidines
- Cyclopentyl compounds
- RTT
- All stub articles
- Antineoplastic and immunomodulating drug stubs