Apixaban
 | |
 | |
| Names | |
|---|---|
| Trade names | Eliquis, others |
| Other names | BMS-562247-01 |
| |
| Clinical data | |
| Drug class | DOAC (direct factor Xa inhibitor)[1] |
| Main uses | Prevent and treat blood clots[1][2] |
| Side effects | Bleeding, nausea[1][2] |
| Routes of use | By mouth |
| Defined daily dose | 10 mg[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613032 |
| Legal | |
| License data | |
| Legal status | |
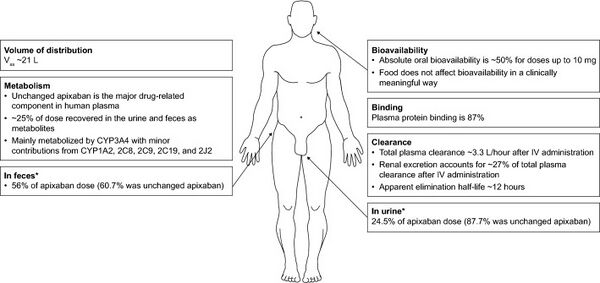
| Pharmacokinetics | |
| Bioavailability | ~50% |
| Protein binding | ~87% |
| Metabolism | CYP3A4, CYP3A5, CYP1A2 and others |
| Elimination half-life | 9–14 h |
| Excretion | Bile (75%), kidney (25%) |
| Chemical and physical data | |
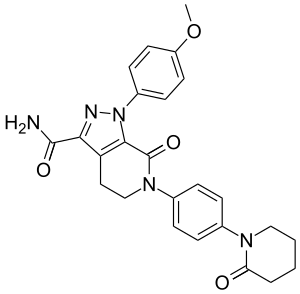
| Formula | C25H25N5O4 |
| Molar mass | 459.497 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Apixaban, sold under the brand name Eliquis among others, is an anticoagulant medication used to treat and prevent blood clots and to prevent stroke in people with nonvalvular atrial fibrillation (AF).[1][2][4] Specifically it is used to prevent blood clots following hip or knee replacement and in those with a history of prior clots.[1][4] It is used as an alternative to warfarin and does not require monitoring by blood tests.[1] It is taken by mouth.[1]
Common side effects include bleeding and nausea.[1][2] Other side effects may include bleeding around the spine and allergic reactions.[1] Use is not recommended during pregnancy or breastfeeding.[2] Use appears to be relatively safe in those with mild kidney problems.[2] Compared to warfarin it has fewer interactions with other medications.[5] It is a direct factor Xa inhibitor.[1]
Apixaban was approved for medical use in the European Union in May 2011 and in the United States in December 2012.[6][7][1] It is on the World Health Organization's List of Essential Medicines as an alternative to dabigatran.[8] A month supply in the United Kingdom costs the NHS about £53 as of 2020.[2] In the United States the wholesale cost of this amount is about $427.[9] In 2017, it was the 93rd most commonly prescribed medication in the United States with more than eight million prescriptions.[10][11] In December 2019, generic versions were approved in the United States.[4]
Medical uses
Apixaban is indicated for the following:[12]
- To lower the risk of stroke and embolism in people with nonvalvular atrial fibrillation.
- Deep vein thrombosis (DVT) prevention. DVTs may lead to pulmonary embolism (PE) in knee or hip replacement surgery patients.
- Treatment of both DVT and PE.
- To reduce the risk of recurring DVT and PE after initial therapy.
In the EU, apixaban is indicated for the prevention of venous thromboembolic events (VTE) in adults who have undergone elective hip or knee replacement surgery, the prevention of stroke and systemic embolism in adults with non-valvular atrial fibrillation (NVAF) with one or more risk factors, for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) in adults, and for the prevention of recurrent DVT and PE in adults.[6]
Atrial fibrillation
Apixaban is recommended by the National Institute for Health and Clinical Excellence for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation and at least one of the following risk factors: prior stroke or transient ischemic attack, age 75 years or older, diabetes mellitus, or symptomatic heart failure.[13]
Apixaban and other anticoagulants (dabigatran, edoxaban and rivaroxaban) appear equally effective as warfarin in preventing non-hemorrhagic stroke in people with atrial fibrillation and are associated with lower risk of intracranial bleeding.[14]
While apixaban may be used in people with severely decreased kidney function and those on hemodialysis it has not been studied in these groups.[1]
Dosage
The defined daily dose is 10 mg by mouth.[3]
| Indication | dose | Comments |
|---|---|---|
| Treatment of VTE | 10mg twice daily for 7 days, then 5mg twice daily | Usually continued for 6 months before changing treatment dose to preventive dose. The dose may need to be halved if taken at the same time as some other common drugs.[1][2] |
| Prevent recurrent VTE | 2.5mg twice daily after 6 months of full treatment | [1][2] |
| Prevent VTE after knee replacement | 2.5mg twice daily for 10-14 days starting 12-24 hours after surgery | Providing any bleeding after surgery has stopped.[1][2] |
| Prevent VTE after hip replacement | 2.5mg twice daily for 32-38 days starting 12-24 hours after surgery | Providing any bleeding after surgery has stopped.[1][2] |
| Non-valvular AF | 5mg twice daily | If one of the following: heart failure, hypertension, previous stroke, TIA, age ≥ 75, diabetes. Half the dose if 2 of the following: age ≥ 80, weight< 61kg, serum creatinine ≥ 133. Half the dose if person is also taking a potent inhibitor of both CYP3A4 and P-gp.[1][2] |
Interactions
The risk of bleeding is increased if apixaban is used at the same time as other blood thinners such as non steroidal anti-inflammatories, anti platelet drugs and heparin, or drugs that are a potent inhibitor of both CYP3A4 and P-gp (eg. ketoconazole, itraconazole, ritonavir).[1][15] The blood thinning effect of apixaban might be reduced with rifampicin and phenytoin.[15]
Side effects
Bleeding
Apixaban can increase the risk of bleeding which may be serious and potentially fatal. Concurrent use with other medications that affect blood clotting can further increase this risk. This includes medications such as other anticoagulants, heparin, aspirin, antiplatelet medications, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and nonsteroidal anti-inflammatory drugs (NSAIDs).[12][16][17][18]
Andexanet alfa is a Food and Drug Administration (FDA) approved antidote for apixaban in people with uncontrolled and life-threatening bleeding events.[19][20]
Spinal puncture
Following spinal anesthesia or puncture people who are being treated with anti-thrombotic agents are at higher risk for developing a hematoma, which can cause long-term or permanent paralysis. The risk of this may be increased by using epidural or intrathecal catheters after a surgical operation or from the concurrent use of medicinal agents that affect hemostasis.[12]
Mechanism of action
Apixaban is a highly selective, orally bioavailable, and reversible direct inhibitor of free and clot-bound factor Xa. Factor Xa catalyzes the conversion of prothrombin to thrombin, the final enzyme in the coagulation cascade that is responsible for fibrin clot formation.[22] Apixaban has no direct effect on platelet aggregation, but by inhibiting factor Xa, it indirectly decreases clot formation induced by thrombin.[12]
History
Apixaban was approved for medical use in the European Union in May 2011.[6]
A new drug application (NDA) for the approval of apixaban was submitted to the U.S. Food and Drug Administration (FDA) by Bristol-Myers Squibb (BMS) and Pfizer jointly after conclusion of the ARISTOTLE clinical trial in 2011.[23][7] Apixaban was approved for the prevention of stroke in people with atrial fibrillation on December 28, 2012.[7][24] On March 13, 2014, it was approved for the additional indication of preventing deep vein thrombosis and pulmonary embolism in people who have recently undergone knee or hip replacement.[25][26] On August 21, 2014, the FDA approved apixaban for the additional indication of the treatment of recurring deep vein thrombosis and pulmonary embolism.[25][27] During its development the drug was known as BMS-562247-01.[28] By late 2019, sales of the product by BMS accounted for thirty-percent of their quarterly revenue.[29] In December 2019, the US FDA approved a generic version produced jointly by Mylan and Micro Labs.[29]
Society and culture
Cost
As of 2020, a month supply of apixaban costs the NHS about £53 in the United Kingdom,[2] where its cost reaches more than 50 times that of warfarin, although this difference may be offset by lower monitoring costs.[15] In the United States the wholesale cost of this amount is about $427.[9] In 2017, it was the 93rd most commonly prescribed medication in the United States with more than eight million prescriptions.[10][11]
-
Apixaban costs (USA)
-
Apixaban prescriptions (USA)
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 "Apixaban Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 27 March 2019. Retrieved 27 March 2019.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 133-134. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ 3.0 3.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 20 September 2020. Retrieved 9 September 2020.
- ↑ 4.0 4.1 4.2 "FDA approves first generics of Eliquis". U.S. Food and Drug Administration (FDA). 23 December 2019. Archived from the original on 23 December 2019. Retrieved 23 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Kiser, Kathryn (2017). Oral Anticoagulation Therapy: Cases and Clinical Correlation. Springer. p. 11. ISBN 9783319546438. Archived from the original on 27 March 2019. Retrieved 27 March 2019.
- ↑ 6.0 6.1 6.2 "Eliquis EPAR". European Medicines Agency. Archived from the original on 11 August 2020. Retrieved 22 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 7.0 7.1 7.2 "Drug Approval Package: Eliquis (apixaban) NDA #202155". U.S. Food and Drug Administration (FDA). 13 February 2013. Archived from the original on 23 December 2019. Retrieved 23 December 2019.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ 9.0 9.1 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ↑ 10.0 10.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 18 March 2020. Retrieved 11 April 2020.
- ↑ 11.0 11.1 "Apixaban - Drug Usage Statistics". ClinCalc. Archived from the original on 28 February 2020. Retrieved 11 April 2020.
- ↑ 12.0 12.1 12.2 12.3 "Eliquis- apixaban tablet, film coated Eliquis 30-day starter pack- apixaban kit". DailyMed. 26 November 2019. Archived from the original on 7 March 2021. Retrieved 22 April 2020.
- ↑ "Apixaban for preventing stroke and systemic embolism in people with nonvalvular atrial fibrillation" (PDF). National Institute for Health and Care Excellence. January 2013. Archived from the original on 5 March 2016. Retrieved 2016-02-26.
- ↑ Gómez-Outes, A; Terleira-Fernández, AI; Calvo-Rojas, G; Suárez-Gea, ML; Vargas-Castrillón, E (2013). "Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups". Thrombosis. 2013: 640723. doi:10.1155/2013/640723. PMC 3885278. PMID 24455237.
- ↑ 15.0 15.1 15.2 Hitchings, Andrew; Lonsdale, Dagan; Burrage, Daniel; Baker, Emma (2019). The Top 100 Drugs: Clinical Pharmacology and Practical Prescribing (2nd ed.). Elsevier. pp. 118–119. ISBN 978-0-7020-7442-4. Archived from the original on 22 May 2021. Retrieved 9 November 2021.
- ↑ "Atrial fibrillation and new oral anticoagulant drugs". U.S. Food and Drug Administration (FDA). 2 December 2015. Archived from the original on 22 April 2020. Retrieved 22 April 2020.
- ↑ "Atrial fibrillation, oral anticoagulant drugs, and their reversal agents". U.S. Food and Drug Administration. 2 December 2015. Archived from the original on 22 April 2020. Retrieved 22 April 2020.
- ↑ "No change is needed in use of direct oral anticoagulants following EMA-funded study". European Medicines Agency (EMA). 27 March 2020. Archived from the original on 22 April 2020. Retrieved 22 April 2020.
- ↑ "Andexxa- andexanet alfa injection, powder, lyophilized, for solution". DailyMed. 8 January 2019. Archived from the original on 18 November 2020. Retrieved 23 December 2019.
- ↑ "Andexxa (coagulation factor Xa (recombinant), inactivated-zhzo)". U.S. Food and Drug Administration (FDA). 31 December 2018. Archived from the original on 29 May 2020. Retrieved 22 April 2020.
- ↑ Byon, Wonkyung; Garonzik, Samira; Boyd, Rebecca A.; Frost, Charles E. (2019). "Apixaban: A Clinical Pharmacokinetic and Pharmacodynamic Review". Clinical Pharmacokinetics. 58 (10): 1265–1279. doi:10.1007/s40262-019-00775-z. ISSN 0312-5963.
- ↑ Frost C, Wang J, Nepal S, et al. (February 2013). "Apixaban, an oral, direct factor Xa inhibitor: single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects". Br J Clin Pharmacol. 75 (2): 476–87. doi:10.1111/j.1365-2125.2012.04369.x. PMC 3558798. PMID 22759198.
- ↑ Granger, Christopher (September 15, 2011). "Apixaban versus Warfarin in Patients with Atrial Fibrillation". New England Journal of Medicine. 365 (11): 981–992. doi:10.1056/NEJMoa1107039. PMID 21870978.
- ↑ Cada, Dennis J.; Levien, Terri L.; Baker, Danial E. (29 May 2013). "Apixaban". Hospital Pharmacy. 48 (6): 494–511. doi:10.1310/hpj4806-494. ISSN 0018-5787. PMC 3839491. PMID 24421512.
- ↑ 25.0 25.1 "FDA-Approved Drugs: Eliquis (apixaban)". U.S. Food and Drug Administration (FDA). Archived from the original on 30 September 2020. Retrieved 23 December 2019.
- ↑ Neale, Todd (March 14, 2014). "FDA OKs Apixaban for DVT Prevention". MedPage Today. Archived from the original on 7 September 2015. Retrieved 17 September 2015.
- ↑ "U.S. FDA Approves Eliquis (apixaban) for the Treatment of Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE), and for the Reduction in the Risk of Recurrent DVT and PE Following Initial Therapy" (Press release). Pfizer. August 21, 2014. Archived from the original on 23 October 2014. Retrieved 2016-02-26.
- ↑ "Apixaban". pubchem.ncbi.nlm.nih.gov. Archived from the original on 3 January 2020. Retrieved 3 January 2020.
- ↑ 29.0 29.1 "FIRST: Mylan, Micro Labs get USFDA nod for generic version of blood thinner Eliquis". Business Medical Dialogues. New Delhi, India: Minerva Medical Treatment. 24 December 2019. Archived from the original on 25 December 2019. Retrieved 24 December 2019.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- CS1 maint: date format
- Wikipedia articles incorporating the PD-notice template
- Drugs with non-standard legal status
- Chem-molar-mass both hardcoded and calculated
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to watched fields
- Bristol-Myers Squibb
- Direct Xa inhibitors
- Lactams
- Pfizer brands
- Phenol ethers
- Piperidines
- Pyrazolopyridines
- RTT
- World Health Organization essential medicines (alternatives)

