Allergic bronchopulmonary aspergillosis
| Allergic bronchopulmonary aspergillosis | |
|---|---|
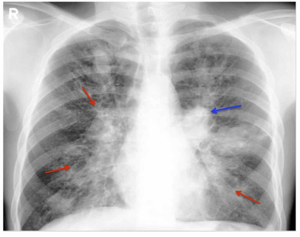 | |
| Chest Xray of allergic bronchopulmonary aspergillosis showing left-sided perihilar opacity (blue arrow) along with non-homogeneous infiltrates (transient pulmonary infiltrates indicated by red arrows) in all zones of both lung fields | |
| Specialty | Infectious disease |
| Symptoms | Wheezing, cough including coughing up blood, fever, tiredness, weight loss[1] |
| Complications | Bronchiectasis[2] |
| Causes | Aspergillus exposure[1] |
| Diagnostic method | Based on symptoms, medical imaging, and blood tests[2][3] |
| Differential diagnosis | Pneumonia, Churg-Strauss syndrome[2] |
| Treatment | Antifungal medication, corticosteroids[1] |
| Frequency | 2% of people with asthma[2] |
Allergic bronchopulmonary aspergillosis (ABPA) is an allergic reaction to the fungus Aspergillus, after it has been inhaled.[1][4] Symptoms may include wheezing, cough including coughing up blood, fever, tiredness, and weight loss.[1] Complications may include bronchiectasis—a condition marked by abnormally dilated airways.[2]
Most people breathe in Aspergillus spores without getting sick.[5] Risk factors include asthma, cystic fibrosis, and being immunocompromised.[3] The underlying mechanism involves inflammation due to an abnormal immune response which results in increased mucus production.[2] The diagnosis may be supported by chest X-ray, CT scan, increased eosinophils, IgE levels of greater than 1,000 IU/mL, or a positive skin allergy test to A. fumigatus.[2] It is a type of aspergillosis.[5]
Treatment is generally with the antifungal medication itraconazole and corticosteroids such as prednisone.[1] Other agents used may include amphotericin B and omalizumab.[2] In about half of people the disease recurs and repeated treatment is requires.[1] ABPA affects about 1 to 4 million people.[4] It occurs in about 2% of people with asthma and up to 15% of people with cystic fibrosis.[2]
Signs and symptoms
Almost all people have asthma,[6] and present with wheezing (usually episodic in nature), coughing, shortness of breath and exercise intolerance (especially in those with cystic fibrosis).[7][8] Moderate and severe cases have symptoms suggestive of bronchiectasis, in particular thick sputum production (often containing brown mucus plugs), as well as symptoms mirroring recurrent infection such as pleuritic chest pain and fever. Patients with asthma and symptoms of ongoing infection, who do not respond to antibiotic treatment, should be suspected of ABPA.[7]
Pathophysiology
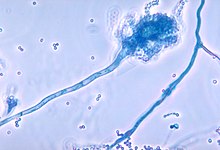
Aspergillus spores are small (2–3 μm in diameter) and can penetrate deep into the respiratory system to the alveolar level.[9][10] In healthy people, innate and adaptive immune responses are triggered by various immune cells (notably neutrophils, resident alveolar macrophages and dendritic cells) drawn to the site of infection by numerous inflammatory cytokines and neutrophilic attractants (such as CXCR2 receptor ligands).[11] In this situation, mucociliary clearance is initiated and spores are successfully phagocytosed, clearing the infection from the host.[9][12]
In people with predisposing lung diseases—such as persistent asthma or cystic fibrosis (or rarer diseases such as chronic granulomatous disease or Hyper-IgE syndrome)—several factors lead to an increased risk of ABPA.[13] These include immune factors (such as atopy or immunogenic HLA-restricted phenotypes),[14][15] as well as genetic factors (such as CFTR gene mutations in both asthmatics and cystic fibrosis patients and a ZNF77 mutation resulting in a premature stop codon in asthmatics and ABPA patients).[16][12][17] By allowing Aspergillus spores to persist in pulmonary tissues, it permits successful germination which leads to hyphae growing in mucus plugs.
There are hypersensitivity responses, both a type I response (atopic, with formation of immunoglobulin E, or IgE) and a type III hypersensitivity response (with formation of immunoglobulin G, or IgG).[12][18] The reaction of IgE with Aspergillus antigens results in mast cell degranulation with bronchoconstriction and increased capillary permeability.[19] Immune complexes (a type III reaction) and inflammatory cells are deposited within the mucous membranes of the airways, leading to necrosis (tissue death) and eosinophilic infiltration.[12] Type 2 T helper cells appear to play an important role in ABPA due to an increased sensitivity to interleukin (IL) 4 and IL-5. These cytokines up-regulate mast cell degranulation, exacerbating respiratory decline.[20][21][22]
Aspergillus also utilises a number of factors to continue evading host responses, notably the use of proteolytic enzymes that interrupt IgG antibodies aimed towards it. Another important feature is its ability to interact and integrate with epithelial surfaces, which results in massive pro-inflammatory counter-response by the immune system involving IL-6, IL-8 and MCP-1 (a CCL2 receptor ligand). Proteases released by both the fungus and neutrophils induce further injury to the respiratory epithelium, leading to initiation of repair mechanisms (such as an influx of serum and extracellular matrix (ECM) proteins) at the site of infection. Aspergillus spores and hyphae can interact with ECM proteins, and it is hypothesised that this process facilitates the binding of spores to damaged respiratory sites.[12][23]
As concentrations of Aspergillus proteases increase, the immunological effect switches from pro-inflammatory to inhibitory, and further reduces phagocytic ability to clear Aspergillus. Ultimately, repeated acute episodes lead to wider scale damage of pulmonary structures (parenchyma) and function via irreversible lung remodelling. Left untreated, this manifests as progressive bronchiectasis and pulmonary fibrosis that is often seen in the upper lobes, and can give rise to a similar radiological appearance to that produced by tuberculosis.[23][24]
Diagnosis
The exact criteria for the diagnosis of ABPA are not yet universally agreed upon, though working groups have proposed specific guidelines.[13][25] Minimal criteria include five factors: the presence of asthma and/or cystic fibrosis, a positive skin test to Aspergillus sp., total serum IgE > 416 IU/mL (or kU/L), an increased Aspergillus species–specific IgE and IgG antibodies, and the presence of infiltrates on a chest X-ray.[26][27]
ABPA should be suspected in patients with a predisposing lung disease—most commonly asthma or cystic fibrosis— and is often associated with chronic airway limitation (CAL). Patients generally present with symptoms of recurrent infection such as fever, but do not respond to conventional antibiotic therapy. Poorly-controlled asthma is a common finding, with a case series only finding 19% of ABPA patients with well-controlled asthma. Wheezing and hemoptysis (coughing up blood) are common features, and mucus plugging is seen in 31–69% of patients.[13]
Blood tests
The first stage involves exposing the skin to Aspergillus fumigatus antigens; an immediate reaction is hallmark of ABPA.[28] The test should be performed first by skin prick testing, and if negative followed with an intradermal injection. The overall sensitivity of the procedure is around 90%, though up to 40% of asthmatic patients without ABPA can still show some sensitivity to Aspergillus antigens (a phenomenon likely linked to a less severe form of ABPA termed severe asthma with fungal sensitization (SAFS)).[13]
Serum blood tests are an important marker of disease severity and are also useful for the primary diagnosis of ABPA. When serum IgE is normal (and patients are not being treated by glucocorticoid medications), ABPA is excluded as the cause of symptoms. A raised IgE increases suspicion, though there is no universally accepted cut-off value. Values can be stated in international units (IU/mL) or ng/mL, where 1 IU is equal to 2.4 ng/mL. Since studies began documenting IgE levels in ABPA during the 1970s, various cut-offs between 833 and 1000 IU/mL have been employed to both exclude ABPA and to warrant further serological testing. The current consensus is that a cut-off of 1000 IU/mL should be employed, as lower values are encountered in SAFS and asthmatic sensitization.[13]
IgG antibody precipitin testing from serum is useful, as positive results are found in between 69 and 90% of patients, though also in 10% of asthmatics with and without SAFS. Therefore, it must be used in conjunction with other tests. Various forms exist, including enzyme-linked immunosorbent assay (ELISA) and fluorescent enzyme immunoassay (FEIA). Both are more sensitive than conventional counterimmunoelectrophoresis. IgG may not be entirely specific for ABPA, as high levels are also found in chronic pulmonary aspergillosis (CPA) alongside more severe radiological findings.[13][29]
Until recently, peripheral eosinophilia (high eosinophil counts) was considered partly indicative of ABPA. More recent studies show that only 40% of ABPA sufferers present with eosinophilia, and hence a low eosinophil count does not necessarily exclude ABPA; for example patients undergoing steroid therapy have lower eosinophil counts.[13]
Medical imaging
Consolidation and mucoid impaction are the most commonly described radiological features described in ABPA literature, though much of the evidence for consolidation comes from before the development of computed tomography (CT) scans. Tramline shadowing, finger-in-glove opacities and ‘toothpaste shadows’ are also prevalent findings.[30]
When utilising high-resolution CT scans, there can be a better assessment of the distribution and pattern of bronchiectasis within the lungs, and hence this is the tool of choice in the radiological diagnosis of ABPA. Central (confined to medial two-thirds of the medial half of the lung) bronchiectasis that peripherally tapers bronchi is considered a requirement for ABPA pathophysiology, though in up to 43% of cases there is a considerable extension to the periphery of the lung.[7]
Mucoid impaction of the upper and lower airways is a common finding.[7] Plugs are hypodense but appear on CT with high attenuation (over 70 Hounsfield units[31]) in up to 20% of patients. Where present it is a strong diagnostic factor of ABPA and distinguishes symptoms from other causes of bronchiectasis.[13]
CT scans may more rarely reveal mosaic-appearance attenuation, centrilobular lung nodules, tree-in-bud opacities and pleuropulmonary fibrosis (a finding consistent with CPA, a disease with ABPA as a known precursor).[7] Rarely other manifestations can be seen on CT scans, including military nodular opacities, perihilar opacities (that mimic hilar lymphadenopathy), pleural effusions and pulmonary masses. Cavitation and aspergilloma are rarer findings, not exceeding 20% of patients, and likely represent a shift from ABPA to CPA if accompanied by pleural thickening or fibrocavitary disease.[13]
-
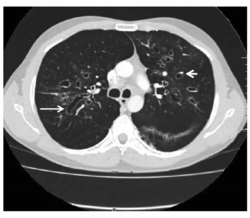
CT showing ‘signet ring’ (short, thick arrow) and ‘string of pearls’ (long, thin arrow) appearances, indicative of central bronchiectasis. Mucoid impaction and dilated bronchi are also seen.
-
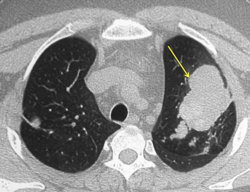
CT of the chest showing high attenuation mucous (HAM) impaction (yellow arrow). This is considered pathognomic for allergic bronchopulmonary aspergillosis (ABPA).
Culture
Culturing fungi from sputum is a supportive test in the diagnosis of ABPA, but is not 100% specific for ABPA as A. fumigatus is ubiquitous and commonly isolated from lung expectorant in other diseases. Nevertheless, between 40 and 60% of patients do have positive cultures depending on the number of samples taken.[13]
Staging
New criteria by the ABPA Complicated Asthma ISHAM Working Group suggests a 6-stage criteria for the diagnosis of ABPA, though this is yet to be formalised into official guidelines.[13] This would replace the current gold standard staging protocol devised by Patterson and colleagues.[25] Stage 0 would represent an asymptomatic form of ABPA, with controlled asthma but still fulfilling the fundamental diagnostic requirements of a positive skin test with elevated total IgE (>1000 IU/mL). Stage 6 is an advanced ABPA, with the presence of type II respiratory failure or pulmonary heart disease, with radiological evidence of severe fibrosis consistent with ABPA on a high-resolution CT scan. It must be diagnosed after excluding the other, reversible causes of acute respiratory failure.[13]
Treatment
Underlying disease must be controlled to prevent exacerbation and worsening of ABPA, and in most this consists of managing their asthma or CF. Any other co-morbidities, such as sinusitis or rhinitis, should also be addressed.[32]
Steroids
Corticosteroids such as prednisone are often used; however, evidence is limited. There is some evidence that acute-onset ABPA is improved by corticosteroid. Concerns with long term corticosteroids include immune dysfunction and metabolic disorders.[32][33]
While the benefits of corticosteroids in the short term are notable, and improve quality of life scores, there are cases of ABPA converting to invasive aspergillosis with this treatment. Furthermore, in concurrent use with itraconazole, there is potential for drug interaction and the induction of Cushing syndrome. Metabolic disorders, such as diabetes mellitus and osteoporosis, can also be induced.[32][33]
In order to mitigate these risks, corticosteroid doses are decreased biweekly assuming no further progression of disease after each reduction. When no exacerbations from the disease are seen within three months after discontinuing corticosteroids, the person is considered to be in complete remission. The exception to this rule is people who are diagnosed with advanced ABPA; in this case, removing corticosteroids almost always results in exacerbation and these patients are continued on low-dose corticosteroids (preferably on an alternate-day schedule).[32][33]
Serum IgE can be used to guide treatment, and levels are checked every 6–8 weeks after steroid treatment commences, followed by every 8 weeks for one year. This allows for a determination of baseline IgE levels, though it's important to note that most patients do not entirely reduce IgE levels to baseline. Chest X-ray or CT scans are performed after 1–2 months of treatment to ensure infiltrates are resolving.[32][33]
Antifungals
Efforts to decrease the use of steroids include using antifungals. The strongest evidence is for itraconazole 200 mg twice daily for four months.[3] Using itraconazole appears to outweigh the risk from long-term and high-dose prednisone. Newer triazole drugs—such as posaconazole or voriconazole—have not yet been studied in-depth.[32][33]
Epidemiology
There are limited national and international studies into the burden of ABPA, made more difficult by a non-standardized diagnostic criteria. Estimates of between 0.5 and 3.5% have been made for ABPA burden in asthma,[34][35] and 1–17.7% in CF.[34][36] Five national cohorts, detecting ABPA prevalence in asthma (based on GINA estimates),[37] were used in a recent meta-analysis to produce an estimate of the global burden of ABPA complicating asthma. From 193 million asthma sufferers worldwide, ABPA prevalence in asthma is estimated between the extremes of 1.35–6.77 million sufferers, using 0.7–3.5% attrition rates. A compromise at 2.5% attrition has also been proposed, placing global burden at around 4.8 million people affected. The Eastern Mediterranean region had the lowest estimated prevalence, with a predicted case burden of 351,000; collectively, the Americas had the highest predicted burden at 1,461,000 cases. These are likely underestimates of total prevalence, given the exclusion of CF patients and children from the study, as well as diagnostic testing being limited in less developed regions.[35]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Allergic bronchopulmonary aspergillosis | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Archived from the original on 19 November 2021. Retrieved 23 January 2022.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "Orphanet: Allergic bronchopulmonary aspergillosis". www.orpha.net. Archived from the original on 23 January 2022. Retrieved 23 January 2022.
- ↑ 3.0 3.1 3.2 Sisodia, Jitendra; Bajaj, Tushar (2022). "Allergic Bronchopulmonary Aspergillosis". StatPearls. StatPearls Publishing. Retrieved 22 January 2022.
- ↑ 4.0 4.1 "Aspergillosis". NORD (National Organization for Rare Disorders). Archived from the original on 21 March 2021. Retrieved 23 January 2022.
- ↑ 5.0 5.1 "About Aspergillosis | Aspergillosis | Types of Fungal Diseases | Fungal Diseases | CDC". www.cdc.gov. 2 February 2021. Archived from the original on 27 June 2021. Retrieved 23 January 2022.
- ↑ Kousha, M.; Tadi, R.; Soubani, A. O. (1 September 2011). "Pulmonary aspergillosis: a clinical review". European Respiratory Review. 20 (121): 156–174. doi:10.1183/09059180.00001011. PMID 21881144. S2CID 38357939.
- ↑ 7.0 7.1 7.2 7.3 7.4 Kousha, M.; Tadi, R.; Soubani, A. O. (1 September 2011). "Pulmonary aspergillosis: a clinical review". European Respiratory Review. 20 (121): 156–174. doi:10.1183/09059180.00001011. PMID 21881144.
- ↑ Greenberger, Paul A. (November 2002). "Allergic bronchopulmonary aspergillosis". Journal of Allergy and Clinical Immunology. 110 (5): 685–692. doi:10.1067/mai.2002.130179. PMID 12417875.
- ↑ 9.0 9.1 Hohl, Tobias M.; Feldmesser, Marta (November 2007). "Aspergillus fumigatus: Principles of Pathogenesis and Host Defense". Eukaryotic Cell. 6 (11): 1953–1963. doi:10.1128/EC.00274-07. PMC 2168400. PMID 17890370.
- ↑ Deacon, L.J.; Pankhurst, L.J.; Drew, G.H.; Hayes, E.T.; Jackson, S.; Longhurst, P.J.; Longhurst, J.W.S.; Liu, J.; Pollard, S.J.T.; Tyrrel, S.F. (November 2009). "Particle size distribution of airborne Aspergillus fumigatus spores emitted from compost using membrane filtration". Atmospheric Environment. 43 (35): 5698–5701. Bibcode:2009AtmEn..43.5698D. doi:10.1016/j.atmosenv.2009.07.042.
- ↑ Dockrell, David H.; McGrath, Emmet E.; Whyte, Moria K.B.; Sabroe, Ian (2007). "The Neutrophil". Immunology of Fungal Infections. pp. 51–73. doi:10.1007/1-4020-5492-0_3. ISBN 978-1-4020-5491-4. S2CID 215264748.
- ↑ 12.0 12.1 12.2 12.3 12.4 Moss, R. B. (January 2005). "Pathophysiology and immunology of allergic bronchopulmonary aspergillosis". Medical Mycology. 43 (s1): 203–206. CiteSeerX 10.1.1.585.3463. doi:10.1080/13693780500052255. PMID 16110813.
- ↑ 13.00 13.01 13.02 13.03 13.04 13.05 13.06 13.07 13.08 13.09 13.10 13.11 Agarwal, R.; Chakrabarti, A.; Shah, A.; Gupta, D.; Meis, J. F.; Guleria, R.; Moss, R.; Denning, D. W.; ABPA complicating asthma ISHAM working group (August 2013). "Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria". Clinical & Experimental Allergy. 43 (8): 850–873. doi:10.1111/cea.12141. PMID 23889240. S2CID 24077597.
- ↑ Antunes, J.; Fernandes, A.; Miguel Borrego, L.; Leiria-Pinto, P.; Cavaco, J. (1 September 2010). "Cystic fibrosis, atopy, asthma and ABPA". Allergologia et Immunopathologia. 38 (5): 278–284. doi:10.1016/j.aller.2010.06.002. hdl:10400.17/1668. PMID 20675033.
- ↑ Muro, Manuel; Mondejar-López, Pedro; Moya-Quiles, María Rosa; Salgado, Gema; Pastor-Vivero, María Dolores; Lopez-Hernandez, Ruth; Boix, Francisco; Campillo, José Antonio; Minguela, Alfredo; Garcia-Alonso, Ana; Sánchez-Solís, Manuel; Álvarez-López, María Rocío (March 2013). "HLA-DRB1 and HLA-DQB1 genes on susceptibility to and protection from allergic bronchopulmonary aspergillosis in patients with cystic fibrosis: HLA and ABPA in cystic fibrosis". Microbiology and Immunology. 57 (3): 193–197. doi:10.1111/1348-0421.12020. PMID 23278646.
- ↑ Marchand, Eric; Delaunois, Luc; Brancaleone, Pierre; Vandenplas, Olivier; Mairesse, Michel; Verellen-Dumoulin, Christine; Rahier, Jean-François (March 2001). "Frequency of Cystic Fibrosis Transmembrane Conductance Regulator Gene Mutations and 5T Allele in Patients With Allergic Bronchopulmonary Aspergillosis". Chest. 119 (3): 762–767. doi:10.1378/chest.119.3.762. PMID 11243954. S2CID 25448045.
- ↑ Gago, Sara; Overton, Nicola L. D.; Ben-Ghazzi, Nagwa; Novak-Frazer, Lilyann; Read, Nick D.; Denning, David W.; Bowyer, Paul (December 2018). "Lung colonization by Aspergillus fumigatus is controlled by ZNF77". Nature Communications. 9 (1): 3835. Bibcode:2018NatCo...9.3835G. doi:10.1038/s41467-018-06148-7. PMC 6147781. PMID 30237437.
- ↑ Chitkara, RK; Sarinas, PS; Fick RB, JR (December 2001). "Immunoglobulin-E and anti-IgE treatment in lung disease". Monaldi Archives for Chest Disease = Archivio Monaldi per le Malattie del Torace. 56 (6): 514–20. PMID 11980283.
- ↑ Mathias, Clinton B.; Freyschmidt, Eva-Jasmin; Caplan, Benjamin; Jones, Tatiana; Poddighe, Dimitri; Xing, Wei; Harrison, Krista L.; Gurish, Michael F.; Oettgen, Hans C. (15 February 2009). "IgE Influences the Number and Function of Mature Mast Cells, but Not Progenitor Recruitment in Allergic Pulmonary Inflammation". The Journal of Immunology. 182 (4): 2416–2424. doi:10.4049/jimmunol.0801569. PMC 2653867. PMID 19201896.
- ↑ Knutsen, A. P.; Hutchinson, P. S.; Albers, G. M.; Consolino, J.; Smick, J.; Kurup, V. P. (January 2004). "Increased sensitivity to IL-4 in cystic fibrosis patients with allergic bronchopulmonary aspergillosis". Allergy. 59 (1): 81–87. doi:10.1046/j.1398-9995.2003.00129.x. PMID 14674938. S2CID 72623230.
- ↑ Müller, U; Piehler, D; Stenzel, W; Köhler, G; Frey, O; Held, J; Grahnert, A; Richter, T; Eschke, M; Kamradt, T; Brombacher, F; Alber, G (May 2012). "Lack of IL-4 receptor expression on T helper cells reduces T helper 2 cell polyfunctionality and confers resistance in allergic bronchopulmonary mycosis". Mucosal Immunology. 5 (3): 299–310. doi:10.1038/mi.2012.9. PMID 22333910.
- ↑ Skov, M; Poulsen, LK; Koch, C (February 1999). "Increased antigen-specific Th-2 response in allergic bronchopulmonary aspergillosis (ABPA) in patients with cystic fibrosis". Pediatric Pulmonology. 27 (2): 74–9. doi:10.1002/(sici)1099-0496(199902)27:2<74::aid-ppul2>3.0.co;2-l. PMID 10088929.
- ↑ 23.0 23.1 Kauffman, Henk F (2003). "Immunopathogenesis of allergic bronchopulmonary aspergillosis and airway remodeling" (PDF). Frontiers in Bioscience. 8 (5): e190–196. doi:10.2741/990. PMID 12456379. S2CID 25768595. Archived from the original (PDF) on 2019-03-05.
- ↑ Collins, J (November 2001). "CT signs and patterns of lung disease". Radiologic Clinics of North America. 39 (6): 1115–35. doi:10.1016/s0033-8389(05)70334-1. PMID 11699664.
- ↑ 25.0 25.1 Patterson, Roy; Greenberger, PA; Radin, RC; Roberts, M (1 March 1982). "Allergic Bronchopulmonary Aspergillosis: Staging as an Aid to Management". Annals of Internal Medicine. 96 (3): 286–291. CiteSeerX 10.1.1.1001.9839. doi:10.7326/0003-4819-96-3-286. PMID 7059089.
- ↑ Reddy, Ashwini; Greenberger, Paul A. (May 2017). "Allergic Bronchopulmonary Aspergillosis". The Journal of Allergy and Clinical Immunology: In Practice. 5 (3): 866–867. doi:10.1016/j.jaip.2016.08.019. PMID 28483324.
- ↑ Knutsen, Alan P.; Bush, Robert K.; Demain, Jeffrey G.; Denning, David W.; Dixit, Anupma; Fairs, Abbie; Greenberger, Paul A.; Kariuki, Barbara; Kita, Hirohito; Kurup, Viswanath P.; Moss, Richard B.; Niven, Robert M.; Pashley, Catherine H.; Slavin, Raymond G.; Vijay, Hari M.; Wardlaw, Andrew J. (February 2012). "Fungi and allergic lower respiratory tract diseases". Journal of Allergy and Clinical Immunology. 129 (2): 280–291. doi:10.1016/j.jaci.2011.12.970. PMID 22284927.
- ↑ Hogan, Celia; Denning, David (December 2011). "Allergic Bronchopulmonary Aspergillosis and Related Allergic Syndromes". Seminars in Respiratory and Critical Care Medicine. 32 (6): 682–692. doi:10.1055/s-0031-1295716. PMID 22167396.
- ↑ Bains, Sonia N.; Judson, Marc A. (June 2012). "Allergic Bronchopulmonary Aspergillosis". Clinics in Chest Medicine. 33 (2): 265–281. doi:10.1016/j.ccm.2012.02.003. PMID 22640845.
- ↑ Greenberger, PA (May–June 2012). "Chapter 18: Allergic bronchopulmonary aspergillosis". Allergy and Asthma Proceedings. 33 Suppl 1 (3): S61–3. doi:10.2500/aap.2012.33.3551. PMID 22794691.
- ↑ Agarwal, Ritesh; Sehgal, Inderpaul Singh; Dhooria, Sahajal; Aggarwal, Ashutosh (2016). "Radiologic Criteria for the Diagnosis of High-Attenuation Mucus in Allergic Bronchopulmonary Aspergillosis". Chest. 149 (4): 1109–1110. doi:10.1016/j.chest.2015.12.043. ISSN 0012-3692. PMID 27055707.
- ↑ 32.0 32.1 32.2 32.3 32.4 32.5 Walsh, Thomas J.; Anaissie, Elias J.; Denning, David W.; Herbrecht, Raoul; Kontoyiannis, Dimitrios P.; Marr, Kieren A.; Morrison, Vicki A.; Segal, Brahm H; Steinbach, William J.; Stevens, David A.; van Burik, Jo-Anne; Wingard, John R.; Patterson, Thomas F.; Infectious Diseases Society of America (1 February 2008). "Treatment of Aspergillosis: Clinical Practice Guidelines of the Infectious Diseases Society of America". Clinical Infectious Diseases. 46 (3): 327–360. doi:10.1086/525258. PMID 18177225.
- ↑ 33.0 33.1 33.2 33.3 33.4 Mahdavinia, Mahboobeh; Grammer, Leslie C. (June 2012). "Management of allergic bronchopulmonary aspergillosis: a review and update". Therapeutic Advances in Respiratory Disease. 6 (3): 173–187. doi:10.1177/1753465812443094. PMID 22547692. S2CID 20436079.
- ↑ 34.0 34.1 Stevens, David A.; Moss, Richard B.; Kurup, Viswanath P.; Knutsen, Alan P.; Greenberger, Paul; Judson, Marc A.; Denning, David W.; Crameri, Reto; Brody, Alan S.; Light, Michael; Skov, Marianne; Maish, William; Mastella, Gianni; Participants in the Cystic Fibrosis Foundation Consensus Conference (October 2003). "Allergic Bronchopulmonary Aspergillosis in Cystic Fibrosis—State of the Art: Cystic Fibrosis Foundation Consensus Conference". Clinical Infectious Diseases. 37 (s3): S225–S264. doi:10.1086/376525. PMID 12975753.
- ↑ 35.0 35.1 Denning, David W.; Pleuvry, Alex; Cole, Donald C. (May 2013). "Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults". Medical Mycology. 51 (4): 361–370. doi:10.3109/13693786.2012.738312. PMID 23210682.
- ↑ Armstead, Joanne; Morris, Julie; Denning, David W. (10 June 2014). "Multi-Country Estimate of Different Manifestations of Aspergillosis in Cystic Fibrosis". PLOS ONE. 9 (6): e98502. Bibcode:2014PLoSO...998502A. doi:10.1371/journal.pone.0098502. PMC 4051580. PMID 24914809.
- ↑ "GINA" (PDF). Global Burden of Asthma. Archived from the original (PDF) on 2013-05-09. Retrieved February 5, 2014.[page needed]
External links
| Classification | |
|---|---|
| External resources |
- Allergic bronchopulmonary aspergillosis Archived 2019-09-28 at the Wayback Machine — GP Notebook