Adalimumab
Adalimumab, sold under the brand name Humira among others, is a medication used to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, psoriasis, hidradenitis suppurativa, uveitis, and juvenile idiopathic arthritis.[1][2][3] Use is generally only recommended in people who have not responded to other treatments.[2] It is used by injection under the skin.[1]
Common side effects include upper respiratory tract infections, pain at the site of injection, rash, and headache.[1] Other side effects may include serious infections, cancer, anaphylaxis, reactivation of hepatitis B, multiple sclerosis, heart failure, liver failure, and aplastic anemia.[1] Use during pregnancy is not recommended, while some feel use during breastfeeding may be safe.[2][6] Adalimumab is a disease-modifying antirheumatic drug and monoclonal antibody that works by inactivating tumor necrosis factor-alpha (TNFα).[1]
Adalimumab was approved for medical use in the United States in 2002.[7][1] It is on the World Health Organization's List of Essential Medicines.[8] It is expensive.[9] A month supply in the United Kingdom costs the NHS about £704 as of 2018.[2] In the United States the wholesale cost of this amount is about US$5,041.[10] In 2014, a biosimilar came to market in India at a price of US$400 per month,[11] and biosimilars are now available in the USA and Europe.[12] In 2017, it was the 169th most commonly prescribed medication in the United States, with more than three million prescriptions.[13][14]
Medical uses
Like other TNF inhibitors, it is an immunosuppressive medication, used to treat autoimmune diseases such as rheumatoid arthritis.[15][16]
Adalimumab is administered by subcutaneous injection.[1][15] For most indications, the maintenance treatment is an injection every other week.[1][15][17]
In the US, adalimumab is indicated for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult crohn's disease, pediatric crohn's disease, ulcerative colitis, plaque psoriasis, hidradenitis suppurativa, and uveitis.[15]
In the EU it is indicated for the treatment of:
- plaque psoriasis (a disease causing red, scaly patches on the skin);[17]
- psoriatic arthritis (a disease causing red, scaly patches on the skin with inflammation of the joints);[17]
- rheumatoid arthritis (a disease causing inflammation of the joints);[17]
- axial spondyloarthritis (inflammation of the spine causing back pain), including ankylosing spondylitis and when X-ray does not show disease but there are clear signs of inflammation;[17]
- polyarticular juvenile idiopathic arthritis and active enthesitis-related arthritis (both rare diseases causing inflammation in the joints);[17]
- Crohn’s disease (a disease causing inflammation of the gut);[17]
- ulcerative colitis (a disease causing inflammation and ulcers in the lining of the gut);[17]
- hidradenitis suppurativa (acne inversa), a long-term skin disease that causes lumps, abscesses (collections of pus) and scarring on the skin;[17]
- non-infectious uveitis (inflammation of the layer beneath the white of the eyeball).[17]
-
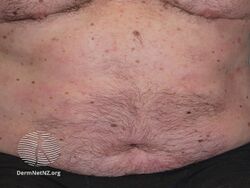
Psoriasis 6 months after commencing adalimumab
-
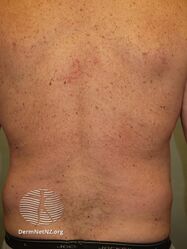
Psoriasis 6 months after commencing adalimumab
-
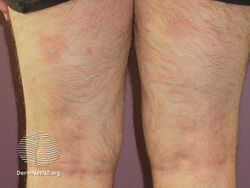
Psoriasis 6 months after commencing adalimumab
Rheumatoid arthritis
Adalimumab has been shown to reduce the signs and symptoms of moderate to severe rheumatoid arthritis in adults. It may be used alone or in combination with disease-modifying antirheumatic drugs (DMARD).[18] It has also been shown to have efficacy in moderate to severe polyarticular juvenile idiopathic arthritis in children four years and older, and is indicated for the treatment of that condition. In rheumatoid athritis, it is indicated for use alone, or with methotrexate or similar medicines, in the United States since 2002.[19][20] It has a similar effectiveness as methotrexate and, in combination, nearly doubles the response rate of methotrexate alone.[21]
Psoriatic arthritis
In 2003, adalimumab began undergoing trials for use in treating psoriasis and psoriatic arthritis.[22]
Ankylosing spondylitis
Adalimumab has been shown to reduce the signs and symptoms of, and is approved for treatment of, ankylosing spondylitis in adults.[23]
Crohn's disease
Adalimumab has been shown to reduce the signs and symptoms of moderate to severe Crohn's disease.[24][12][25] It has been approved for that use in the UK since 2009.[26]
Ulcerative colitis
Adalimumab may be effective and well tolerated in ulcerative colitis. It was approved by the US Food and Drug Administration (FDA) for treatment of moderate to severe cases in adults.[27][28]
Plaque psoriasis
Adalimumab has been shown to treat moderate to severe chronic plaque psoriasis in adults who have the condition in many areas of their body and who may benefit from taking injections or pills (systemic therapy) or phototherapy (treatment using ultraviolet light alone or with pills).[29] Adalimumab has been shown to be effective therapy when used either continuously or intermittently in patients with moderate to severe psoriasis.[30]
Hidradenitis suppurativa
Adalimumab was approved for hidradenitis suppurativa in 2015.[3][31][32]
Juvenile idiopathic arthritis
Adalimumab has been shown to reduce the signs and symptoms of moderate to severe polyarticular juvenile idiopathic arthritis in children aged four years and older.[33][34][35]
Non-infectious uveitis
Adalimumab is indicated for the treatment of non-infectious uveitis (inflammation of the layer beneath the white of the eyeball).[17][15]
Dosage
The defined daily dose is 2.9 mg by injection.[5]
Side effects
There is strong evidence that adalimumab increases risk of serious infections, such as tuberculosis. It also increases the risk of cancers, including lymphoma and solid malignancies. The risk of cancer is higher with higher doses of adalimumab.[36]
There are rare reports of serious liver injury; rare reports of demyelinating central nervous system disorders; and rare reports of cardiac failure—the US Food and Drug Administration (FDA) issued a black box warning to doctors, which appears in the product labeling of adalimumab and other TNF-inhibiting drugs, instructing them to screen and monitor potential patients more carefully.[37]
Anaphylaxis or other serious allergic reactions may also occur.[37]
History
Adalimumab was the first fully human monoclonal antibody approved by the US Food and Drug Administration (FDA).[38] It was derived from phage display.[39][38]
Adalimumab was discovered as a result of a collaboration between BASF Bioresearch Corporation and Cambridge Antibody Technology, U.K., itself a collaboration of the government-funded Medical Research Council and three academics, which began in 1993.[40][41][38]
Initially named D2E7,[42] it was then further manufactured at BASF Bioresearch Corporation, developed by BASF Knoll (BASF Pharma), and ultimately manufactured and marketed by Abbott Laboratories after Abbott's acquisition of BASF Pharma. On 1 January 2013, Abbott split into two companies, one retaining the Abbott name and the other named AbbVie.[43] As a result, AbbVie took over development and marketing of Humira.[44][45] The brand name Humira stands for "human monoclonal antibody in rheumatoid arthritis", and was named by one of Abbott's employees, Richard J. Karwoski, who was also responsible for leading the effort to get Humira approved by the FDA.
It was the third TNF inhibitor, after infliximab and etanercept, to be approved in the United States.[38] It was constructed from a fully human monoclonal antibody, while infliximab is a mouse-human chimeric antibody and etanercept is a TNF receptor-IgG fusion protein.[medical citation needed]
The drug candidate was discovered initially using CAT's phage display technology and named D2E7.[42] The key components of the drug were found by guiding the selection of human antibodies from phage display repertoires to a single epitope of an antigen TNF alpha.[46] The ultimate clinical candidate, D2E7, was created and manufactured at BASF Bioresearch Corporation and taken through most of the drug development process by BASF Knoll, then further development, manufacturing and marketing by Abbott Laboratories, after Abbott acquired the pharmaceutical arm of BASF Knoll.[47]
Since 2008, adalimumab had been approved by the FDA for the treatment of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, moderate to severe chronic psoriasis and juvenile idiopathic arthritis. Although only approved for ulcerative colitis from late 2012, by the FDA in the disease's management, it had been used for several years in cases that have not responded to conventional treatment at standard dosing for Crohn's disease.[citation needed]
Adalimumab, sold under the brand name Humira, was approved for use in the United States in 2002.[7][48]
Adalimumab, sold under the brand names Humira and Trudexa, was approved for use in the European Union in September 2003.[17][49]
-
Removing the caps from the pen
-
Adalimumab pen
-
Components of a Humira autoinjector pen.
Marketing
- 1999: Preliminary results of early clinical trials with the fully human anti-TNFα monoclonal antibody D2E7[42]
- 2001, June: Results from ARMADA, a double-blind, placebo-controlled clinical trial involving 271 patients with active rheumatoid arthritis despite treatment with methotrexate are announced. Among the results are that 50% of patients show a 50% improvement in American College of Rheumatology (ACR) score.[50]
- 2002: Broke ground on a new state-of-the-art biologics manufacturing facility.[51]
- 2002: Adalimumab results from five separate trials show that it is effective at reducing signs and symptoms of rheumatoid arthritis. In these studies, adalimumab had a rapid onset of action and sustained efficacy. Furthermore, adalimumab was safe and effective when given alone or in combination with MTX as a subcutaneous injection.[52]
- 2002, 31 December: Humira approved by the US Food and Drug Administration (FDA) for treatment of rheumatoid arthritis.[51]
- 2003: Launched Humira for rheumatoid arthritis and continued clinical studies for additional indications.[51]
- 2005: Launched Humira for psoriatic arthritis. Exceeded US$1 billion in annual sales for the first time.[51]
- 2005: Eisai Submits New Drug Application for Rheumatoid Arthritis Drug Adalimumab (D2E7) in Japan.[53]
- 2006: Submitted Humira for the Crohn's disease indication and launched it for AS. Exceeded US$2 billion in annual sales.[51]
- 2007: Launched Humira for Crohn's disease in the United States,[51] submitted Humira for global regulatory approval for psoriasis — the fifth new Humira disease indication at this time, achieved more than US$3 billion in worldwide Humira sales.[54]
- 2007: Abbott Opens New Biotechnology Manufacturing Facility in Puerto Rico[55]
- 2008: Launched Humira for plaque psoriasis[56]
- 2009: Five-Year Data Demonstrate Initial Use of Humira Plus Methotrexate May Prevent Further Joint Damage in Early Rheumatoid Arthritis Patients [57][58]
- 2012: Humira could be associated with a significant decrease in vascular inflammation, a major risk factor of cardiovascular disease [59]
- 2013: Due to the split of Abbott, Humira rights are now owned by AbbVie.[43][44]
- 2014: Humira recognized by IMS Health as the "world's best selling drug."[60]
- 2014: In December 2014, Indian drugmaker Cadila Healthcare declared the launch of the first adalimumab biosimilar at a fifth of its US price. The generic was launched under the brand name Exemptia.[11]
- 2015: Launched Humira for moderate to severe hidradenitis suppurativa, an orphan indication. No other treatment has been[when?] rigorously tested and found to be safe and effective in treating this painful and scarring condition.[3]
- 2016: The best selling drugs list researched by Genetic Engineering & Biotechnology News, published in March 2017, details that Humira occupied the #1 position for 2015 (US$14.012 billion) and 2016 (US$16.078 billion)[61]
- 2017: AbbVie reports that Humira achieved US$18.427 billion of sales in 2017.[62]
Society and culture
Costs
From 2012, until the US patent expiry in 2016, Humira led the list of top-selling pharmaceutical products, and in 2016, it had US$16 billion of global sales.[63] In 2017, it was the 169th most commonly prescribed medication in the United States, with more than three million prescriptions.[13] In 2014, a biosimilar came to market in India at a lower price compared to United States prices.[11]
-
Adalimumab prescriptions in US
-
Adalimumab costs (USA)
Royalty litigation
In March 2003, Cambridge Antibody Technology (CAT) stated its wish to "initiate discussions regarding the applicability of the royalty offset provisions for Humira" with Abbott Laboratories in the High Court of London. In November 2004, the trial began, and in December 2004, Justice Hugh Laddie ruled for CAT.
A short version of the full statement of the proceedings was released.[64] In it Justice Laddie remarked, "Abbott was in error when it made its first royalty payment to CAT calculated on the basis that only 2% of the Net Sales was due. It should have calculated on the basis of the full royalty of just over 5% and should have paid and continued to pay CAT accordingly." Justice Laddie went on to observe "...that the construction advanced by Abbott does violence to the language of the agreements, renders them obscure and makes little or no commercial sense. For this reason CAT wins the action."[65]
Abbott was required to pay CAT US$255 million, some of which was to be passed to its partners in development.[66] Of this sum, the Medical Research Council received US$191 million, and in addition, Abbott was asked to pay the MRC a further US$7.5 million over five years from 2006, providing that Humira remains on the market. The MRC also is to receive a further £5.1 million (sterling) in respect of past royalties.[67]
Patent litigation
On 29 May 2009, Johnson & Johnson's Centocor unit, the maker of infliximab, won a ruling for $1.67 billion from Abbott Laboratories for patent infringement on the process for making Humira.[68] However, in 2011, the judgment was overturned by the United States Court of Appeals for the Federal Circuit.[69][70][71]
Biosimilars
In 2014, Indian drugmaker Cadila Healthcare declared the launch of the first adalimumab biosimilar at a fifth of its US price. The generic was launched under the brand name Exemptia.[11] In 2016, Indian drugmaker Torrent Pharmaceuticals launched its biosimilar for adalimumab, called Adfrar. It was the second generic biosimilar of adalimumab.[72]
In 2016, the FDA approved Amgen's biosimilar adalimumab-atto, sold under the brand name Amjevita.[73][74][75] Amjevita won't be available in the US until at least February 2023.[76] In 2017, the FDA approved German pharmaceutical company Boehringer Ingelheim's biosimilar, Cyltezo.[77][78][79]
In 2017, the biosimilars Amgevita,[80] Solymbic,[81] Imraldi,[82] and Cyltezo[83] were approved for use in the European Union.
In 2018, the biosimilars Halimatoz,[84] Hefiya,[85] Hyrimoz,[86] and Hulio[87] were approved for use in the European Union.
Adalimumab biosimilars became available in Europe in late 2018,[88] allowing the National Health Service to make record-breaking cost-savings,[89] as this is the single most expensive drug used in NHS hospitals, costing more than £400 million a year for about 46,000 patients.[90] It may not become available in the United States until 2023.[88][91]
In 2018, adalimumab-adaz (Hyrimoz) was approved for use in the United States.[92][93]
In April 2019, Idacio[94] and Kromeya[95] were approved for use in the European Union.
In July 2019, adalimumab-bwwd (Hadlima), produced by Samsung Bioepsis, was approved for use in the US.[96][97] However, it will not be available until at least June 2023, after the availability of Amgen's offering as a result of a negotiated intellectual property settlement with AbbVie.[98]
In November 2019, adalimumab-afzb (Abrilada) was approved for use in the United States.[99][100] It was the 25th biosimilar to be approved by the FDA.[101]
In February 2020, the biosimilar Amsparity was approved for use in the European Union.[102]
In July 2020, adalimumab-fkjp (Hulio) was approved for use in the United States.[103]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Adalimumab Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. 14 May 2018. Archived from the original on 21 November 2020. Retrieved 18 March 2019.
- ↑ 2.0 2.1 2.2 2.3 2.4 British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 1074. ISBN 9780857113382.
- ↑ 3.0 3.1 3.2 3.3 "FDA Clears Adalimumab (Humira) for Hidradenitis Suppurativa". Medscape. 11 September 2015. Archived from the original on 21 January 2021. Retrieved 13 October 2017.
- ↑ 4.0 4.1 Use During Pregnancy and Breastfeeding
- ↑ 5.0 5.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 31 October 2020. Retrieved 7 September 2020.
- ↑ "Adalimumab Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 17 April 2021. Retrieved 19 March 2019.
- ↑ 7.0 7.1 "Drug Approval Package: Humira (adalimumab)". U.S. Food and Drug Administration (FDA). 7 April 2017. Archived from the original on 18 February 2020. Retrieved 18 February 2020.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ Hitchings, Andrew; Lonsdale, Dagan; Burrage, Daniel; Baker, Emma (2022). "Monoclonal antibodies". The Top 100 Drugs (3rd ed.). Elsevier Health Sciences. pp. 166–167. ISBN 978-0-323-83445-2. Archived from the original on 19 September 2023. Retrieved 18 September 2023.
- ↑ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ↑ 11.0 11.1 11.2 11.3 "India's Cadila launches first cheaper copy of world's top-selling drug". Reuters. 9 December 2014. Archived from the original on 27 March 2019. Retrieved 19 March 2019.
- ↑ 12.0 12.1 Peyrin-Biroulet L, Danese S, Cummings F, Atreya R, Greveson K, Pieper B, et al. (26 July 2019). "Anti-TNF biosimilars in Crohn's Disease: a patient-centric interdisciplinary approach". Expert Review of Gastroenterology & Hepatology. 13 (8): 731–738. doi:10.1080/17474124.2019.1645595. ISSN 1747-4124. PMID 31322440.
- ↑ 13.0 13.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 18 March 2020. Retrieved 11 April 2020.
- ↑ "Adalimumab - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
- ↑ 15.0 15.1 15.2 15.3 15.4 "Humira- adalimumab kit Humira- adalimumab injection, solution". DailyMed. Archived from the original on 21 June 2020. Retrieved 18 February 2020.
- ↑ "Imraldi- adalimumab injection, solution". DailyMed. 23 January 2018. Archived from the original on 21 September 2020. Retrieved 18 February 2020.
- ↑ 17.00 17.01 17.02 17.03 17.04 17.05 17.06 17.07 17.08 17.09 17.10 17.11 "Humira EPAR". European Medicines Agency (EMA). Archived from the original on 18 February 2020. Retrieved 18 February 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ Navarro-Sarabia F, Ariza-Ariza R, Hernandez-Cruz B, Villanueva I (20 July 2005). "Adalimumab for treating rheumatoid arthritis". The Cochrane Database of Systematic Reviews (3): CD005113. doi:10.1002/14651858.CD005113.pub2. PMID 16034967.
- ↑ Siegel, Jay P. "Product Approval Information - Licensing Action". U.S. Food and Drug Administration (FDA). Archived from the original on 24 October 2020. Retrieved 4 February 2014.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Humira (Adalimumab)" (PDF). Archived (PDF) from the original on 19 March 2013. Retrieved 4 February 2014.
- ↑ Welch, Beth (15 December 2008). "Adalimumab (Humira) for the Treatment of Rheumatoid Arthritis". American Family Physician. 78 (12): 1406–1408. ISSN 0002-838X. Archived from the original on 24 November 2020. Retrieved 9 September 2019.
{{cite journal}}: Unknown parameter|name-list-format=ignored (help) - ↑ Scheinfeld N (2003). "Adalimumab (Humira): a review". J Drugs Dermatol. 2 (4): 375–7. PMID 12884458.
- ↑ Maxwell LJ, Zochling J, Boonen A, Singh JA, Veras MM, Tanjong Ghogomu E, et al. (18 April 2015). "TNF-alpha inhibitors for ankylosing spondylitis". The Cochrane Database of Systematic Reviews. 4 (4): CD005468. doi:10.1002/14651858.CD005468.pub2. PMID 25887212. Archived from the original on 20 September 2020. Retrieved 14 December 2019.
- ↑ Podolsky DK (August 2002). "Inflammatory bowel disease" (PDF). N Engl J Med. 347 (6): 417–29. doi:10.1056/NEJMra020831. PMID 12167685. Archived (PDF) from the original on 14 March 2020. Retrieved 24 September 2019.
- ↑ Gearry RB, Frampton C, Inns S, Poppelwell D, Rademaker M, Suppiah R (3 July 2019). "VITALITY: impact of adalimumab on health and disability outcomes in patients with Crohn's disease, rheumatoid arthritis, or psoriasis treated in clinical practice in New Zealand". Current Medical Research and Opinion. 35 (10): 1837–1846. doi:10.1080/03007995.2019.1634952. ISSN 0300-7995. PMID 31233347.
- ↑ Morey, Sheralyn (17 September 2009). "UK – Summary of NICE Approvals in September 2009". Archived from the original on 22 February 2014. Retrieved 4 February 2014.
- ↑ "FDA approves Humira to treat ulcerative colitis" (Press release). U.S. Food and Drug Administration (FDA). 28 September 2012. Archived from the original on 30 September 2012.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Abbott's Humira (adalimumab) Receives U.S. FDA Approval for the Treatment of Adult Patients with Moderate to Severe Ulcerative Colitis". Abbott (Press release). 28 September 2012. Archived from the original on 19 November 2019. Retrieved 18 November 2019.
- ↑ Croom KF, McCormack PL (2009). "Adalimumab". Am J Clin Dermatol. 10 (1): 43–50. doi:10.2165/0128071-200910010-00008. PMID 19170412.
- ↑ Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, Strober BE, Kaul M, Gu Y, Okun M, Papp K (January 2008). "Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial". J. Am. Acad. Dermatol. 58 (1): 106–15. doi:10.1016/j.jaad.2007.09.010. PMID 17936411.
- ↑ Gulliver W, Zouboulis CC, Prens E, Jemec GB, Tzellos T (September 2016). "Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa". Rev Endocr Metab Disord. 17 (3): 343–351. doi:10.1007/s11154-016-9328-5. PMC 5156664. PMID 26831295.
- ↑ Maarouf M, Clark AK, Lee DE, Shi VY (August 2018). "Targeted treatments for hidradenitis suppurativa: a review of the current literature and ongoing clinical trials". J Dermatolog Treat. 29 (5): 441–449. doi:10.1080/09546634.2017.1395806. PMID 29098911.
- ↑ Brunner HI, Nanda K, Toth M, Foeldvari I, Bohnsack J, Milojevic D, Rabinovich CE, Kingsbury DJ, Marzan K, Chalom E, Horneff G, Kuester RM, Dare JA, Trachana M, Jung LK, Olson J, Minden K, Quartier P, Bereswill M, Kalabic J, Kupper H, Lovell DJ, Martini A, Ruperto N (August 2019). "Safety and Effectiveness of Adalimumab in Patients With Polyarticular Course of Juvenile Idiopathic Arthritis: STRIVE Registry 7-Year Interim Results". Arthritis Care Res (Hoboken). doi:10.1002/acr.24044. PMID 31421019.
- ↑ Horneff G, Seyger MM, Arikan D, Kalabic J, Anderson JK, Lazar A, Williams DA, Wang C, Tarzynski-Potempa R, Hyams JS (October 2018). "Safety of Adalimumab in Pediatric Patients with Polyarticular Juvenile Idiopathic Arthritis, Enthesitis-Related Arthritis, Psoriasis, and Crohn's Disease". J. Pediatr. 201: 166–175.e3. doi:10.1016/j.jpeds.2018.05.042. PMID 30054164.
- ↑ Correll CK, Bullock DR, Cafferty RM, Vehe RK (February 2018). "Safety of weekly adalimumab in the treatment of juvenile idiopathic arthritis and pediatric chronic uveitis". Clin. Rheumatol. 37 (2): 549–553. doi:10.1007/s10067-017-3890-4. PMID 29103180.
- ↑ Hochman D, Wolff B (November 2006). "Risk of serious infections and malignancies with anti-TNF antibody therapy in rheumatoid arthritis". JAMA. 296 (18): 2203, author reply 2203–4. doi:10.1001/jama.296.18.2203-a. PMID 17090763.
- ↑ 37.0 37.1 "Humira Medication Guide" (PDF). October 2018. Archived (PDF) from the original on 13 November 2008. Retrieved 9 September 2019.
- ↑ 38.0 38.1 38.2 38.3 Frenzel A, Schirrmann T, Hust M (October 2016). "Phage display-derived human antibodies in clinical development and therapy". MAbs. 8 (7): 1177–94. doi:10.1080/19420862.2016.1212149. PMC 5058633. PMID 27416017.
- ↑ Brekke OH, Sandlie I (January 2003). "Therapeutic antibodies for human diseases at the dawn of the twenty-first century". Nat Rev Drug Discov. 2 (1): 52–62. doi:10.1038/nrd984. PMID 12509759.
- ↑ [1][permanent dead link]
- ↑ McCafferty J (2010). "The long and winding road to antibody therapeutics". mAbs. 2 (5): 459–460. doi:10.4161/mabs.2.5.13088. ISSN 1942-0862. PMC 2958567. PMID 20978369.
- ↑ 42.0 42.1 42.2 Kempeni J (January 1999). "Preliminary results of early clinical trials with the fully human anti-TNFα monoclonal antibody D2E7". Ann Rheum Dis. 58 (suppl 1): I70–2. doi:10.1136/ard.58.2008.i70. PMC 1766582. PMID 10577977.
- ↑ 43.0 43.1 "Abbott Completes Separation Of Research-Based Pharmaceuticals Business" (Press release). Abbott Laboratories. Archived from the original on 8 August 2020. Retrieved 9 September 2019.
- ↑ 44.0 44.1 Turner, Sally (5 September 2018). "Humira: the highs and lows of the world's most successful drug". Pharmaceutical Technology. Archived from the original on 9 September 2019. Retrieved 9 September 2019.
- ↑ Japsen, Bruce (26 February 2019). "Why Abbvie May Have A Tough Time Defending Humira's Price Before Congress". Forbes. Archived from the original on 27 February 2019. Retrieved 9 September 2019.
- ↑ Jespers LS, Roberts A, Mahler SM, Winter G, Hoogenboom HR (September 1994). "Guiding the selection of human antibodies from phage display repertoires to a single epitope of an antigen". Bio/Technology. 12 (9): 899–903. doi:10.1038/nbt0994-899. PMID 7521646.
- ↑ "BASF to focus more strongly on innovative chemistry, a highly efficient Verbund and a global presence" (Press release). Archived from the original on 12 February 2013. Retrieved 9 December 2012.
- ↑ "Humira: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 18 February 2020. Retrieved 18 February 2020.
- ↑ "Trudexa EPAR". European Medicines Agency (EMA). Archived from the original on 18 February 2020. Retrieved 18 February 2020.
- ↑ "Abbott Laboratories' Investigational Fully Human Anti-Tnf Therapy, D2E7 (Adalimumab), Shows Promise In Reducing The Signs And Symptoms Of Rheumatoid Arthritis" (Press release). 12 November 2001. Archived from the original on 4 December 2019. Retrieved 9 September 2019.
- ↑ 51.0 51.1 51.2 51.3 51.4 51.5 "Abbott 2006 Annual Report" (PDF). Abbott. Archived from the original on 13 January 2008. Retrieved 27 July 2009.
- ↑ Rau R (January 2002). "Adalimumab (a fully human anti-tumour necrosis factor α monoclonal antibody) in the treatment of active rheumatoid arthritis: the initial results of five trials". Ann Rheum Dis. 61 (Suppl 2): ii70–3. doi:10.1136/ard.61.suppl_2.ii70. PMC 1766697. PMID 12379628.
- ↑ "Eisai Submits New Drug Application for Rheumatoid Arthritis Drug Adalimumab (D2E7) in Japan". thefreelibrary.com. Archived from the original on 13 October 2012. Retrieved 27 July 2009.
- ↑ "Abbott 2007 Annual Report" (PDF). Abbott. Archived from the original on 3 May 2009. Retrieved 27 July 2009.
- ↑ "Abbott Opens New Biotechnology Manufacturing Facility In Puerto Rico" (Press release). Abbott. 10 April 2007. Archived from the original on 30 May 2009. Retrieved 27 July 2009.
- ↑ "Abbott's Humira (adalimumab) Receives FDA Approval For Moderate to Severe Chronic Plaque Psoriasis" (Press release). Abbott Laboratories. 18 January 2008. Archived from the original on 7 August 2020. Retrieved 9 September 2019.
- ↑ "Five-Year Data Demonstrate Initial Use Of Abbott's Humira (Adalimumab) Plus Methotrexate May Prevent Further Joint Damage In Early Rheumatoid Arthritis Patients" (Press release). Abbott. Archived from the original on 22 March 2010. Retrieved 27 July 2009.
- ↑ Papagoras C, Voulgari PV, Drosos AA (2009). "Long-term use of adalimumab in the treatment of rheumatic diseases". Open Access Rheumatology: Research and Reviews. 1: 51–68. doi:10.2147/oarrr.s4297. ISSN 1179-156X. PMC 5074727. PMID 27789981.
- ↑ "Treating psoriasis to prevent heart attacks and strokes". ScienceDaily. Archived from the original on 20 September 2018. Retrieved 9 March 2018.
- ↑ "Humira Lifts AbbVie 2.8% in Q2". Discovery and Development. Associated Press. 25 July 2014. Archived from the original on 27 July 2014. Retrieved 27 July 2014.
- ↑ "The Top 15 Best-Selling Drugs of 2016 - The Lists - GEN". Genengnews.com. Archived from the original on 8 March 2018. Retrieved 8 March 2018.
- ↑ "AbbVie Reports Full-Year and Fourth-Quarter 2017 Financial Results". AbbVie (Press release). Archived from the original on 12 April 2018. Retrieved 8 March 2018.
- ↑ "The Top 15 Best-Selling Drugs of 2016 - The Lists - GEN Genetic Engineering & Biotechnology News - Biotech from Bench to Business - GEN". Genengnews.com. Archived from the original on 8 March 2017. Retrieved 8 March 2017.
- ↑ [2][permanent dead link]
- ↑ "Biotech firm wins royalty fight". BBC News Online. 20 December 2004. Archived from the original on 27 August 2021. Retrieved 23 April 2010.
- ↑ Murray-West, Rosie (27 October 2005). "Drug maker CAT surges after royalty settlement". The Telegraph. London. Archived from the original on 20 September 2018. Retrieved 23 April 2010.
- ↑ "Multi-Million Dollar Deal To Benefit Medical Research" (PDF) (Press release). Medical Research Council. Archived from the original (PDF) on 27 June 2007. Retrieved 20 July 2009.
- ↑ Pierson, Ransdell; Spicer, Jonathan (30 June 2009). "Jury returns $1.67 billion drug verdict against Abbott". Reuters. Archived from the original on 2 March 2021. Retrieved 9 September 2019.
- ↑ "Centocor Ortho Biotech v. Abbott Laboratories, 636 F. 3d 1341". Court of Appeals, Federal Circuit 2011. 23 February 2011. Archived from the original on 27 August 2021. Retrieved 9 September 2019 – via Google Scholar.
- ↑ "Centocor Ortho Biotech Inc V. Abbott Laboratories". FindLaw. 23 February 2011. Archived from the original on 3 March 2011. Retrieved 9 September 2019.
- ↑ Decker, Susan (23 February 2011). "Abbott Wins Reversal of J&J's $1.67 Billion Patent Victory". Bloomberg Businessweek. Archived from the original on 6 May 2011.
- ↑ "Torrent launches world's second biosimilar of generic auto-immune drug". Business Standard. 11 January 2016. Archived from the original on 26 February 2016. Retrieved 1 March 2016.
- ↑ "Drug Approval Package: Amjevita (adalimumab-atto)". U.S. Food and Drug Administration (FDA). 9 November 2016. Archived from the original on 4 October 2020. Retrieved 11 February 2020.
- ↑ "FDA approves Amjevita, a biosimilar to Humira". U.S. Food and Drug Administration (FDA) (Press release). 23 September 2016. Archived from the original on 19 November 2019. Retrieved 18 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Amjevita: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 18 February 2020. Retrieved 18 February 2020.
- ↑ Stanton, Dan (3 October 2017). "Humira biosimilar settlement a 'win-win' for Amgen and AbbVie, lawyer". Biopharma-Reporter. Archived from the original on 27 August 2021. Retrieved 18 February 2020.
- ↑ "Drug Approval Package: Cyltezo (adalimumab-adbm)". U.S. Food and Drug Administration (FDA). 27 September 2018. Archived from the original on 4 October 2020. Retrieved 11 February 2020.
- ↑ "Cyltezo: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 18 February 2020. Retrieved 18 February 2020.
- ↑ "Boehringer Ingelheim Pharmaceuticals, Inc. receives FDA approval for Cyltezo (adalimumab-adbm), a biosimilar to Humira, for the treatment of multiple chronic inflammatory diseases" (Press release). Boehringer Ingelheim Pharmaceuticals. 29 August 2017. Archived from the original on 24 July 2019. Retrieved 19 March 2019.
- ↑ "Amgevita EPAR". European Medicines Agency (EMA). Archived from the original on 30 December 2019. Retrieved 18 February 2020.
- ↑ "Solymbic EPAR". European Medicines Agency (EMA). Archived from the original on 25 June 2019. Retrieved 18 February 2020.
- ↑ "Imraldi EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 24 March 2020. Retrieved 18 February 2020.
- ↑ "Cyltezo EPAR". European Medicines Agency (EMA). Archived from the original on 6 August 2020. Retrieved 18 February 2020.
- ↑ "Halimatoz EPAR". European Medicines Agency (EMA). Archived from the original on 30 December 2019. Retrieved 18 February 2020.
- ↑ "Hefiya EPAR". European Medicines Agency (EMA). Archived from the original on 15 October 2019. Retrieved 18 February 2020.
- ↑ "Hyrimoz EPAR". European Medicines Agency (EMA). Archived from the original on 16 November 2019. Retrieved 18 February 2020.
- ↑ "Hulio EPAR". European Medicines Agency (EMA). Archived from the original on 24 March 2020. Retrieved 18 February 2020.
- ↑ 88.0 88.1 Hargreaves, Ben (16 October 2018). "First wave of Humira biosimilars enters EU market". BioPharma-Reporter. Archived from the original on 15 August 2020. Retrieved 26 November 2018.
- ↑ Heal, Alexandra (26 November 2018). "NHS replaces highest-spend drug with £300m cheaper alternative". The Guardian. Archived from the original on 26 November 2018. Retrieved 26 November 2018.
- ↑ "Most expensive NHS drug comes off patent". Pharmaceutical Journal. 18 October 2018. Archived from the original on 21 February 2019. Retrieved 29 November 2018.
- ↑ Calandra, Robert; Huff, Charlotte; Butcher, Lola (25 January 2019). "Bringing Humira (Its Price) Down a Peg". Managed Care magazine. Archived from the original on 8 January 2020. Retrieved 9 September 2019.
- ↑ "Hyrimoz: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). 30 October 2018. Archived from the original on 7 December 2019. Retrieved 6 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Drug Approval Package: Hyrimoz". U.S. Food and Drug Administration (FDA). 21 March 2019. Archived from the original on 18 December 2019. Retrieved 11 February 2020.
- ↑ "Idacio EPAR". European Medicines Agency (EMA). Archived from the original on 30 December 2019. Retrieved 18 February 2020.
- ↑ "Kromeya EPAR". European Medicines Agency (EMA). Archived from the original on 30 December 2019. Retrieved 18 February 2020.
- ↑ "Hadlima: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 19 October 2020. Retrieved 8 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Drug Approval Package: Hadlima". U.S. Food and Drug Administration (FDA). 5 September 2019. Archived from the original on 18 December 2019. Retrieved 11 February 2020.
- ↑ "FDA approves Humira biosimilar, but it won't hit the market for 4 years". Becker's Hospital Review. 24 July 2019. Archived from the original on 8 November 2019. Retrieved 8 November 2019.
- ↑ "Abrilada: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). 15 November 2019. Archived from the original on 7 December 2019. Retrieved 6 December 2019.
- ↑ "Drug Approval Package: Abrilada". U.S. Food and Drug Administration (FDA). 28 January 2020. Archived from the original on 4 October 2020. Retrieved 11 February 2020.
- ↑ "Statement from Sarah Yim, M.D., acting director of the Office of Therapeutic Biologics and Biosimilars in the FDA's Center for Drug Evaluation and Research, on FDA's continued progress facilitating competition in the biologic marketplace with approval of 25th biosimilar product". U.S. Food and Drug Administration (FDA). 15 November 2019. Archived from the original on 19 November 2019. Retrieved 18 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Amsparity EPAR". European Medicines Agency (EMA). Archived from the original on 18 February 2020. Retrieved 18 February 2020.
- ↑ "Drugs@FDA: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 7 July 2020. Retrieved 7 July 2020.
External links
| Identifiers: |
|
|---|
- "Adalimumab". Drug Information Portal. US National Library of Medicine. Archived from the original on 12 February 2020. Retrieved 19 November 2019.
- Pages using duplicate arguments in template calls
- Pages with non-numeric formatnum arguments
- Wikipedia articles incorporating the PD-notice template
- CS1 errors: unsupported parameter
- All articles with dead external links
- Articles with dead external links from October 2016
- Articles with invalid date parameter in template
- Articles with permanently dead external links
- Articles with dead external links from September 2017
- Use dmy dates from September 2019
- Chem-molar-mass both hardcoded and calculated
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugs that are a monoclonal antibody
- All articles with unsourced statements
- Articles with unsourced statements from September 2019
- All articles with vague or ambiguous time
- Vague or ambiguous time from November 2019
- Articles with hatnote templates targeting a nonexistent page
- Chemicals that do not have a ChemSpider ID assigned
- Articles with changed EBI identifier
- AbbVie brands
- Immunosuppressants
- Monoclonal antibodies
- TNF inhibitors
- World Health Organization essential medicines
- RTT